Tables and Figures referenced in the processing description are found in the paper Frank et al., 2015
Potential energy yields of the different metabolisms available in the incubations depend on temperature and fluid compositions. To quantify the energy yield from heterotrophic sulfate reduction (Table 2) in each incubation values of overall Gibbs energy () were calculated according to:
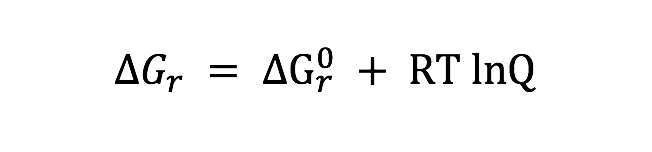
where is the standard Gibbs energy of reaction at in situ temperature and pressure conditions, R is the gas constant, T is the temperature (Kelvin), and Q is the activity product, defined as
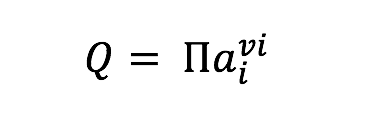
where ai represents the activity of the ith species and vi is the stoichiometric reaction coefficient, which is positive for products and negative for reactants. Values of were calculated at 1 bar and incubation temperatures using the geochemical software package SUPCRT92 (Johnson et al., 1992) and additional thermodynamic data from (Shock, 1995). Activities of aqueous species were calculated using the geochemical speciation program EQ3 (Wolery, 1992) based on the media composition described in section 2.2 and Table 1, with additional data from previously published work (Shock, 1995; Shock & Koretsky, 1993). For concentrations equal to zero, a value of 10-13 mol/kg was used as input. Resulting aqueous activities were used to calculate values of normalized for the number of electrons transferred in the redox for the reactions in Table 2. These reflect the metabolic energy available at the start of each incubation experiment for the complete oxidation of each organic acid, metabolisms that are documented among known sulfate reducers (Amend and Shock, 2001). Furthermore, to calculate the energy density in each incubation (as in Amend et al., 2011), it was assumed that the amended organic acids were the limiting reactant for all experiments when sulfate concentrations were in excess of 1 mM; otherwise sulfate was assumed to be limiting. While some sulfate reducers are known to produce carboxylic acid and alcohol intermediates, incomplete oxidation reactions were not considered here, as the goal of these calculations was to generate a broad understanding of sulfate reduction energetics, and not the metabolic potential for a particular species. Such an approach is common when comparing microbial metabolisms independent of species-specific pathways (e.g. Amend et al., 2004; Rogers & Amend, 2006; Skoog et al., 2007), although it should be noted that incomplete oxidation (fermentation) generally yields much less energy than complete oxidation (Rogers & Amend, 2006; Skoog et al., 2007).
To account for potential interactions between chimney-derived trace metals and amended sulfide, the saturation states of sulfide minerals were calculated as part of the initial fluid speciation. Using reported concentrations of relevant trace metals (Fe, Zn, Cu, etc.) in end-member Grotto hydrothermal fluid (Butterfield et al., 1994), maximum aqueous activities of trace metals were calculated with the EQ3 geochemical speciation program (EQ3/6 1998; EQ3NR 1998). Several sulfide minerals commonly found in hydrothermal chimneys (e.g. pyrite, chalcocite, sphalerite) were supersaturated under incubation conditions, particularly for incubations with high concentrations of amended sulfide. The irreversible abiotic precipitation of mineral sulfides has the potential to draw down aqueous sulfide concentrations and impact sulfate reductions rates. Therefore, the geochemical reaction path program EQ6 (EQ3/6 1998; EQ6 1998) was used to constrain fluid compositions to equilibrium with these minerals phases. Using the single point model in EQ6, the Gibbs energy of the system was allowed to reach local minima by mineral precipitation, however redox reactions among carbon and sulfur species was suppressed with a custom thermodynamic database. The resulting fluid compositions were used to calculate metabolic reaction energetics as well as to evaluate the potential effects of metal speciation on sulfate reduction rates.
BCO-DMO Data Processing Notes:
-reformatted column names to comply with BCO-DMO standards
-filled in all blank cells with nd
-removed spaces and replaced with underscores

 ©2025 Biological and Chemical Oceanography Data Management Office.
©2025 Biological and Chemical Oceanography Data Management Office.
