Niskin bottle hydrography from the CTD rosette from cruises KN193-03, B4-2008, B9-2008, and B10-2008 from the subpolar North Atlantic and Iceland Basin in 2008 (NAB 2008 project)
Project
Program
| Contributors | Affiliation | Role |
|---|---|---|
| Perry, Mary Jane | University of Maine (U Maine DMC) | Principal Investigator |
| Cetinic, Ivona | University of Maine (U Maine DMC) | Contact |
| McKee, Theresa | Woods Hole Oceanographic Institution (WHOI BCO-DMO) | BCO-DMO Data Manager |
This dataset includes nutrients, pigment concentrations, cell counts and composition, backscatter and spectral absorption coefficients for particles and phytoplankton.
Modification history:
30 March 2011:
Corrected time parameter (hhmm) caused by scripting error during conversion from Matlab to BCO-DMO
29 March 2011:
variable bbp700 upgraded to six decimal places from four to increase resolution
07 January 2011:
Four variables removed at P.I. request:
ap748; ap765; aph748; aph765
10 May 2011
KN193-03 bottle data updated from April 2011 newly-submitted data.
Variable changed were:
allox,bac_het_cyt,carotene,chl_a,chl_a2,chl_a_tot,chl_a_tot_mol,chl_b,chl_b2,chl_c1_c2,chl_c3,chlide_a,
ciliates,coccus_s_cyt,crypto,diadinox,diatom,diatox,dino_auto_mix,fucox,fucox_but,gyrox,lutein,
nanoflag_het,neox,p_phide_a,p_phytin_a,peridinin,phaeo,phyto_oth,pnans,prasinox,violax,zeax
Acquisition details from each cruise are reported for that platform deployment.
Data processing and analysis details from each cruise are reported for that platform deployment.
| File |
|---|
Niskin.csv (Comma Separated Values (.csv), 557.03 KB) MD5:2f75a60a552cdf8d37f99c7c9d9199e9 Primary data file for dataset ID 3393 |
| Parameter | Description | Units |
| Cruise_ID | deployment designation as in other files | dimensionless |
| cast | CTD cast number (not station number) | integer |
| date | date (GMT) YYYYMMDD (year; month; day) | YYMMDD |
| time | time (GMT) HHMM (hours; minutes) | HHMM |
| lat | latitude | decimal degrees |
| lon | longitude | decimal degrees |
| depth_bottom | seafloor bottom depth | meters |
| beamc_id | C-Star serial number [284 or 1090] | integer |
| yrday | time when sample was taken in decimal days since Jan-0-0000 (Matlab) | dimensionless |
| depth | depth at which sample was taken | meters |
| temp | temperature | degrees Celsius |
| sal | salinity | dimensionless |
| sigma_0 | water potential density minus 1000 | kilograms/meter^3 |
| O2_Winkler | dissolved oxygen concentration by Winkler method | micromol/kilogram |
| O2_cal | dissolved oxygen concentration; calibrated with Winkler O2 throughout cruise | micromol/kilogram |
| O2_uncal | dissolved oxygen concentration; not calibrated during cruise with Winkler O2 | micromol/kilogram |
| NO3_NO2 | nitrate plus nitrite | micromol/liter |
| POC | particulate organic carbon | milligramsreciprocal meter^3 |
| PAR | water column photosynthetically active radiation (PAR) | micromoles photon/reciprocal meter^2/second |
| SPAR | surface photosynthetically active radiation (sPAR) | micromol photon/reciprocal meter^2/second |
| bbp700 | particulate backscattering coefficient (bbp) | reciprocal meter |
| beam_cp | particulate attenuation coefficient (cp) | reciprocal meter |
| beamc_id | C-Star serial number [284 or 1090] | integer |
| Si_acid | silicic acid | micromoles liter |
| allox | HPLC Alloxanthin | micrograms/liter |
| ap412 | particulate absorption coefficient at 412 nm | reciprocal meter |
| ap443 | particulate absorption coefficient at 443 nm | reciprocal meter |
| ap488 | particulate absorption coefficient at 488 nm | reciprocal meter |
| ap490 | particulate absorption coefficient at 490 nm | reciprocal meter |
| ap510 | particulate absorption coefficient at 510 nm | reciprocal meter |
| ap531 | particulate absorption coefficient at 531 nm | reciprocal meter |
| ap551 | particulate absorption coefficient at 551 nm | reciprocal meter |
| ap555 | particulate absorption coefficient at 555 nm | reciprocal meter |
| ap667 | particulate absorption coefficient at 667 nm | reciprocal meter |
| ap670 | particulate absorption coefficient at 670 nm | reciprocal meter |
| ap678 | particulate absorption coefficient at 678 nm | reciprocal meter |
| aph412 | phytoplankton absorption coefficient at 412 nm | reciprocal meter |
| aph443 | phytoplankton absorption coefficient at 443 nm | reciprocal meter |
| aph488 | phytoplankton absorption coefficient at 488 nm | reciprocal meter |
| aph490 | phytoplankton absorption coefficient at 490 nm | reciprocal meter |
| aph510 | phytoplankton absorption coefficient at 510 nm | reciprocal meter |
| aph531 | phytoplankton absorption coefficient at 531 nm | reciprocal meter |
| aph551 | phytoplankton absorption coefficient at 551 nm | reciprocal meter |
| aph555 | phytoplankton absorption coefficient at 555 nm | reciprocal meter |
| aph667 | phytoplankton absorption coefficient at 667 nm | reciprocal meter |
| aph670 | phytoplankton absorption coefficient at 670 nm | reciprocal meter |
| aph678 | phytoplankton absorption coefficient at 678 nm | reciprocal meter |
| carotene | HPLC alpha (eta-epsilon) + beta (beta-beta) Carotenes | micrograms/liter |
| chl_a | HPLC Chlorophyll a | micrograms/liter |
| chl_a2 | HPLC Divinyl Chlorophyll a | micrograms/liter |
| chl_a_fluor | chlorophyll a - fluorometric analysis of acetone extract | milligrams/meter^3 |
| chl_a_tot | HPLC total chlorophyll a; (HPLC Chl_a + Chlide_a) | micrograms/liter |
| chl_a_tot_mol | HPLC total Chlorophyll a (Chl_a + Chlide_a) molar concentration | micromol/meter^3 |
| chl_b | HPLC Chlorophyll b | micrograms/liter |
| chl_b2 | HPLC Divinyl Chlorophyll b | micrograms/liter |
| chl_c1_c2 | HPLC Chlorophyll c1 + c2 | micrograms/liter |
| chl_c3 | HPLC Chlorophyll c3 | micrograms/liter |
| chl_raw | chlorophyll fluorescence (raw output minus dark counts) | volts |
| chlide_a | HPLC Chlorophyllide a | micrograms/liter |
| ciliates | chlorophyll-containing ciliate abundance | cells/milliliter |
| ciliates_C | chlorophyll-containing Ciliates carbon | micrograms carbonliter |
| coccus_s_cyt | Synechococcus abundance | cells/milliliters |
| coccus_s_cyt_C | Synechococcus carbon | micrograms carbon/liter |
| crypto | cryptophyte abundance | cells/milliliter |
| crypto_C | cryptophyte carbon | micrograms carbon/liter |
| diadinox | HPLC Diadinoxanthin | micrograms/liter |
| diatox | HPLC Diatoxanthin | micrograms per liter |
| neox | HPLC Neoxanthin | micrograms/liter |
| p_phorbide_a | HPLC Pheophorbide a | micrograms/liter |
| p_phytin_a | HPLC Pheophytin a | micrograms/liter |
| peridinin | HPLC Peridinin | micrograms/liter |
| phaeo | pheopigment - fluorometric analysis of acetone extract | milligrams/meter^3 |
| fucox | HPLC Fucoxanthin | micrograms/liter |
| fucox_but | HPLC 19'-Butanoyloxyfucoxanthin | micrograms/liter |
| gyrox | HPLC Gyroxanthin-Diester | micrograms/liter |
| lutein | HPLC Lutein | micrograms/liter |
| prasinox | HPLC Prasinoxanthin | micrograms/liter |
| violax | HPLC Violaxanthin | micrograms/liter |
| zeax | HPLC Zeaxanthin | micrograms/liter |
| bac_het_cyt | heterotrophic bacteria abundance | cells/milliliter |
| bac_het_cyt_C | heterotrophic bacteria carbon | micrograms carbon/liter |
| diatom | diatom containing image abundance | images containing diatoms per liter |
| diatom_C | Diatom carbon | micrograms carbon/liter |
| nanoflag_het | heterotrophic nanoflagellate abundance | cells/milliliter |
| nanoflag_het_C | heterotrophic nanoflagellates carbon | micrograms carbon/liter |
| dino_auto_mix | autotrophic and mixotrophic dinoflagellate abundance | cells/milliliter |
| dino_auto_mix_C | Autotrophic and mixotrophic dinoflagellate carbon | micrograms carbon/liter |
| phyto_oth | microphytoplankton abundance for other chlorophyll-containing particles not classified as diatoms, dinoflagellates or ciliates | chlorophyll-containing microplankton per liter |
| phyto_oth_C | microphytoplankton carbon for other chlorophyll-containing particles not classified as diatoms or dinoflagellates or ciliates | micrograms carbon/liter |
| MICROP_Total_C | total microphytoplankton carbon (sum of diatom_C + dino_auto_mix_C + ciliate_C + phyto_oth_C) | micrograms carbon/liter |
| NANOP_Total_C | sum of cell carbon for photosynthetic picoeukaryotes + Synechococcus + cryptophytes (pnans + coccus_s_cyt + crypto) | micrograms carbon/liter |
| Dataset-specific Instrument Name | Spectrophotomer-Varian Cary 50UV |
| Generic Instrument Name | Spectrophotomer-Varian Cary 50UV |
| Generic Instrument Description | The Varian Cary 50 UV-Visible Spectrophotometer has a xenon flash lamp and a 1.5nm slit width for measurement of total particulate absorption spectra. |
KN193-03
| Website | |
| Platform | R/V Knorr |
| Start Date | 2008-05-01 |
| End Date | 2008-05-22 |
| Description | A three-week process cruise on the R/V Knorr operated in the vicinity of five autonomous platforms that had been deployed in early April by another vessel. A total of 10 simultaneous float and CTD calibration profiles were taken to calibrate sensors on the Lagrangian mixed layer float (Biofloat 48) and to validate proxy measurements (i.e., optical attenuation to particulate organic carbon, etc.). One simultaneous Seaglider and CTD calibration profile was collected for each of the four Seagliders. Knorr also carried out a number of bow-tie surveys around the Lagrangian mixed layer float. A second float, Biofloat 47, had ceased functioning shortly after deployment was rescued at the beginning of the cruise. Two SOLOPC floats were deployed but were damaged on deployment and sank. A number of successful short deployments of PELAGRA floating were made during the cruise.
Core ship-board measurements supported by project funding were: 1) CTD profiles (temperature, conductivity, oxygen, chlorophyll fluorescence, optical backscatter, and beam transmission) on all four cruises; 133 CTD profiles were obtained on this cruise. 2) analysis of water samples collected with the CTD Rosette (chlorophyll, HPLC pigments, nutrients, particulate organic carbon, particulate absorption spectrum, phytoplankton, oxygen and other guest investigator measurements).
Original cruise data are available from the NSF R2R data catalog
Science personnel:
Mary Jane Perry, University of Maine, Chief Scientist
Witold Bagniewsk, University of Maine
Nicole Bale, Plymouth Laboratory, UK
Nathan Briggs, University of Maine
David Checkley, Scripps Institution of Oceanography
Giorgio Dall'Olmo, Oregon State University
Andrea Drzewianowski, University of Maine
Amanda Gray, University of Washington
Jennifer Fortier, University of Maine
Alba Gonzalez-Posada, University of East Anglia, UK
Emily Kallin, University of Maine
Kristinn Gudmundsson, Marine Research Institute, Reykjavik, Iceland
Richard Lampitt, National Oceanography Centre, South Hampton, UK
Patrick Martin, National Oceanography Centre, South Hampton, UK
Maren Moltke Lyngsgaard, University of Copenhagen, Denmark
Nicole Poulton, Bigelow Laboratory
Eric Rehm, University of Washington
Katherine Richardson, University of Copenhagen, Denmark
Ryan Rykaczewski, Scripps Institution of Oceanography
Michael Sauer, University of Maine
Richard Sawyer, National Oceanography Centre, South Hampton, UK
Michael Sieracki, Bigelow Laboratory
Tatiana Rynearson, University of Rhode Island
Toby Westberry, Oregon State University
Dane Wojcicki, University of Maine
Lin Zhang, University of Rhode Island Methods & Sampling The WHOI CTD Rosette on the R/V Knorr was equipped with the following sensors. Sensor owner is identified in parenthesis (WHOI or Perry): Sea-Bird Electronics 11+ CTD deck unit (WHOI); Sea-Bird Electronics 9+ CTD with Dual SBE3T/SBE4C temperature/conductivity sensors (WHOI); 24-bottle Rosette with 10-liter bottles (WHOI); Sea-Bird Electronics 43 oxygen sensor (WHOI); WET Labs ECO FLNTU, measuring chlorophyll fluorescence with excitation at 470 nm and emission at 695 nm and volume scattering function at 140° and 700 nm (Perry); WET Labs C-Star transmissometer, measuring transmission at 653 nm (s/n 284, loaned to Perry; s/n 1090, Perry); WET Labs CDOM ECO fluorometer – measuring colored dissolved organic material (CDOM) fluorescence with excitation at 370 nm and emission at 460 nm (loaned to Perry); Seapoint Turbidity Meter measuring volume scattering function over a wide angle (15 – 150º) at 880 nm (WHOI); Biospherical Instruments underwater QSP2300 sensor, measuring scalar underwater photosynthetically active radiation (PAR; Perry); Biospherical Instruments surface QSR-240 Quantum Scalar Reference Sensor providing surface photosynthetically active radiation (sPAR; WHOI). For more details, see sensor set up and instrument calibration factors document (Knorr19303_PsaReport.txt) contributed by original investigators. CTD data are reported as cast number and not as station number. CTD Rosette system profiled at 0.5 m s-1 between the surface and 200 m, and at 1 m s-1 below 200 m. Bottles were fired on the upcasts, 60 s after the CTD stopped. Sensor data are averages of a 30-s stationary period immediately before the bottle was fired. On CTD casts 16, 17, 59, 61, 63, 82, 109 and 124, the pump associated with the Oxygen SBE43 sensor failed, resulting in partial or total removal of oxygen data from the dataset (parameter name O2_cal). For those casts with pump failure, temperature and salinity data from the second CT sensors were used. WET Labs C-Star s/n 284 was used on cruise B200804 (deployment cruise on R/S Bjarni Saemundsson) and the first part of KN19303 (process cruise). It flooded and was replaced with C-Star s/n 1090 for the remainder of the process cruise (it was also used on the last R/S Bjarni Saemundsson cruise, B200810). Data (parameter name beam_cp) associated with malfunctioning and flooded C-Star s/n 284 have been removed from following casts: 13, 25–30 and 43. The parameter beamc_id indicates which C-Star was used (s/n 284 or 1090). Cross calibration of C-Stars across different cruises is discussed below, under Data Processing. Processing Description Temperature and salinity (parameter names temp and sal): The CTD had dual CT sensors. For profiles where the two sensors agreed, a 51- (2.1 s) point median filter was applied to the mean of the two sensors. For profiles or regions of the profile where the two sensors disagreed, data from the sensor with the lesser variability was chosen and a 101-point (4.2 s) median filter was applied to only that sensor. Further manual smoothing was performed for a few casts. For more details on temperature and salinity processing, see Ship_TS_despiking-NAB08.pdf Dissolved oxygen, O2 (parameter name O2_cal): The sensor was calibrated immediately before the cruise. The factory calibration was applied to SBE43 oxygen sensor output and data were converted to µmol kg-1 and aligned with Winkler O2 measurements (included in bottle file, KN19303_bottle_file.mat). A time-dependent quadratic correction was applied to the SBE data, with resulting measurement error of 3.2 µmol kg-1. For more details, see Oxygen_Calibration-NAB08.pdf Particulate attenuation coefficient, cp (parameter name beam_cp): The factory calibration was used to convert C-Star s/n 1090 voltage to particulate attenuation coefficient, cp (m-1); the calibration factor did not change between pre-cruise and post-cruise factory calibration. C-Star s/n 284 was cross calibrated with C-Star s/n 1090 through a series of simultaneous ship/Biofloat48 calibration profiles. The final products for all three C-Stars are cross-calibrated cp coefficients with units of m-1. Complete details of the transmissometer intercalibration procedure are in C-Star calibration report (C-Star_Calibration-NAB08.pdf). Particulate backscattering coefficient, bbp (parameter name bbp700): Backscattering voltage was converted to β at 140° by subtracting dark voltages (median in-situ dark voltage, 0.078 V) and multiplying by factory calibration scale factors, modified based on measurements and calculations of Sullivan et al. (subm.). The calibration factor did not change between pre-cruise and post-cruise factory calibration. β at 140° was converted to bbp (m-1) by subtracting β of seawater (Zhang et al., 2009) and multiplying by 2πχ (where χ = 1.132). See calibration report for more details: Backscatter_Calibration-NAB08.pdf [Note on conversion of bbp700 to POC: Ship-based bbp estimates over the entire experiment were ~20% higher on the CTD downcasts than the upcasts. This systematic difference may be due to the optical properties of aggregates (Briggs, 2010), but remains under investigation. Stationary bbp measurements and POC contained in this bottle file cannot be directly applied to the downcast bbp measurements in the CTD data sets. However, a correction is included in the CTD data sets (KN19303_castXXX_1m_down.mat and KN19303_castXXX_down.mat) that converts CTD bbp from the downcast to POC. For more details, see POC_cp_bbp_Calibration-NAB08.pdf.] Chlorophyll fluorescence (parameter name chl_raw) is reported as the raw instrument voltage output minus dark counts (median in situ dark voltage = 0.083 volts). Surface photosynthetically active radiation (parameter name SPAR): The sensor was new and calibrated before the cruise. Calibration coefficients for surface PAR sensor were incorrect in the original SBE configuration file. The incorrect coefficients were removed in the post processing and correct coefficients were applied to the data. For more details, see Radiometry_and_PAR_Calibration-NAB08.pdf Water column photosynthetically active radiation (parameter name PAR): Reported values for water column PAR might have a positive bias of ~0.08 µmol photon m^-2 s^-1 based on discrepancies between factory calibration sheet and value used in the SBE configuration file. This error is very small and usually less than or similar to calibration associated error, as noted in the factory calibrations. The sensor was new and freshly calibrated before the 1 April cruise on the R/S Bjarni Saemundsson (cruise B200804). For more details, see Radiometry_and_PAR_Calibration-NAB08.pdf Dissolved oxygen, O2 (parameter name O2_Winkler): Water samples were collected and pickled immediately after Niskin bottles were brought on deck. Samples were analyzed with the Winkler method, using visual determination of the titration endpoint, following WOCE (Culberson, 1991) and JGOFS (Knap et al., 1996) procedures. For additional details on laboratory analysis of discrete water samples see: Laboratory_analysis_report-NAB08.pdf Nutrients (parameter names NO3_NO2 and Si_acid): Nutrient samples for nitrate plus nitrite (NO3-+ NO2-) and silicic acid (Si(OH4)) were collected in acid-washed LDPE bottles; unfiltered samples were frozen immediately and stored for up to 8 mo. Samples were thawed at room temperature in the dark for 24 h and vigorously vortexed (Gordon et al., 1993). Samples were analyzed with a Lachat Quickchem 8000 Flow Injection Analysis System using standard absorptiometric techniques (Smith and Bogren, 2001; Wolters, 2002; QuikChemâMethod 31-107-04-1-C for nitrate plus nitrite; QuikChemâMethod 31-114-27-1-B for silicic acid). All Lachat spectra were manually inspected for irregularities in baseline or the presence of bubbles. Any offending samples were rejected. Profiles of silicic acid and nitrate concentrations for all casts were also examined, following the recommendation of the IODE workshop on quality control of chemical oceanographic data (IOC, 2010). Concentrations that were clearly out of range without temperature or salinity intrusions were rejected. Phosphate baselines as a whole were not sufficiently stable to be accepted and hence phosphate data are not reported. For additional details on laboratory analysis of discrete water samples see: Laboratory_analysis_report-NAB08.pdf Fluorometric pigments (parameter names chl_a_fluor and phaeo): Water samples for fluorometric analysis of chlorophyll and pheopigments were filtered through Whatman GF/F filters. Triplicate water samples were collected at 10 m. Filters were extracted in 5 ml of 90% acetone at -20° C for 24 h and read on a Turner Designs Model 10-AU Digital fluorometer. The fluorometer was calibrated before and after the field program with Turner Designs chlorophyll standards. Chlorophyll and pheopigment were determined following JGOFS protocol procedures (Knap et al., 1996). For additional details on laboratory analysis of discrete water samples see: Laboratory_analysis_report-NAB08.pdf Particulate organic carbon (parameter name POC): Samples were collected from Niskin bottles using a sampling bell to minimize particle contamination from air. They were immediately filtered, using closed bottles fitted with Biochem Fluidics caps with 2 tubing ports, through a Millipore Swinnex in-line filter holder onto pre-combusted Whatman GF/F filters. All plastics were washed in RBS. Samples were stored frozen for up to 5 mo. Before analysis, samples were dried at 50 °C for 4 h, fumed with hydrochloric acid (HCl, 11.65 N) for 12 h, and stored in a desiccator for up to 12 h. Filters were rolled and placed in pre-combusted tin cups shortly before analysis on a Perkin Elmer 2400CHN analyzer (Knap et al., 1996). For additional details on laboratory analysis of discrete water samples see:Laboratory_analysis_report-NAB08.pdf HPLC pigment samples (see various parameter names): Water samples were filtered onto Whatman GF/F filters and preserved in -80°C (liquid nitrogen) until analysis. Samples were stored for up to 5 mo. HPLC analysis was performed by Horn Point laboratories using a methanol-based reversed-phase gradient C8 chromatography column system and appropriate standards (Hooker et al., 2009; Van Heukelem and Thomas, 2001). For additional details on laboratory analysis of discrete water samples see: Laboratory_analysis_report-NAB08.pdf Particulate and phytoplankton absorption coefficients (parameter names ap(λ) and aph(λ): Water samples for absorption spectra were filtered onto Whatman GF/F filters and scanned at sea on a Varian Cary 50 UV-Visible Spectrophotometer with a xenon flash lamp and a 1.5nm slit width for measurement of total particulate absorption spectra, ap(λ) (Mitchell and Kiefer, 1988). Filters were subsequently extracted in methanol and re-analyzed to determine residual detrital particulate absorption, ad(λ); the difference spectra are reported as phytoplankton absorption spectra, aph(λ) (Kishino et al., 1985). For additional details on laboratory analysis of discrete water samples see: Laboratory_analysis_report-NAB08.pdf Plankton cell counts (various parameter names designated with broad taxonomic group name): Plankton cell numbers were determined at sea. Picophytoplankton were analyzed with a FACScan flow cytometer with chlorophyll a and phycoerythrin fluorescence as discriminators for three groups: photosynthetic eukaryotic nanophytoplankton (parameter name pnans), Synechococcus (parameter name coccus_s_cyt), and cryoptophytes (parameter name crypto). Heterotrophic bacteria (parameter name bac_het_cyt) were stained with PicoGreen (Veldhuis et al. 1997) and heterotrophic nanoprotists (parameter name nanoflag_het) were stained with LysoTracker Green (Rose et al., 2004) before flow cytometric analysisMicroplankton digital images of single cells, chains and colonies were collected with a FlowCAM; image collection was triggered by chlorophyll a fluorescence. Microplankton were classified into four super classes: 1) diatoms (parameter name diatom), including centrics, pennates, Guinardia, Thallasionema, Rhizosolenia and Chaetoceros); 2) photosynthetic and mixotrophic dinoflagellates (parameter name dino_auto_mix, including Ceratium and Dinophysis); 3) chlorophyll-containing ciliates (parameter name ciliate); and 4) other chlorophyll-containing microplankton not classified as diatoms, dinoflagellates or ciliates (parameter name phyto_oth). Dinoflagellates and ciliates are reported as cells per liter. Since diatoms and other microplankton are not always present as individual cells (e.g., diatom chains), units reported are number of images containing diatoms or chlorophyll-containing microplankton per liter; these images may contain a single cell, a chain of cells or a colony.For additional details on phytoplankton analysis from discrete water samples see: Phytoplankton_Carbon-NAB08.pdf Plankton carbon (various parameter names designated with broad taxonomic group name_C): Plankton cell carbon was determined from cell counts and volumes at sea. Picophytoplankton, bacteria and heterotrophic nanoprotists were counted as described for flow cytometric plankton cell counts. Cell size for all these groups was determined from forward scatter. Size and scatter relationships were established with standard microbeads and algal cultures using a Coulter Counter. Cell carbon was estimated from cell size using the biomass algorithm of Verity et al. (1992). Microplankton data were collected with a FlowCAM as described under plankton cell counts. Biovolumes were determined for each of four groups listed under plankton cell counts, based on Sieracki et al. (1989). Carbon was computed based on Menden-Deuer and Lessard (2000). For additional details see: Phytoplankton_Carbon-NAB08.pdf. |
B4-2008
| Website | |
| Platform | R/V Bjarni Saemundsson |
| Start Date | 2008-04-01 |
| End Date | 2008-04-06 |
| Description | Deployment cruise:
R/S Bjarni Saemundsson departed 1 April 2008 10:00 from Reykjavik to deploy 2 floats (Biofloat 47 and 48 and 4 Seagliders (SG 140, 141, 142, 143); these were all successfully deployed on 4 April 2008. Biofloat 47 failed within a few weeks of deployment; therefore its data are not reported.
CTD profiles (n=9) and water samples were collected before and after the autonomous platform deployment. R/S Bjarni Saemundsson returned to Reykjavik on 6 April 2008. Methods & Sampling CTD Rosette deployed from the R/S Bjarni Saemundsson was equipped with following sensors: NOTE: sensor owner is identified in parenthesis (Marine Research Institute or Perry). Sea-Bird Electronics 11+ CTD deck unit (Marine Research Institute); Sea-Bird Electronics 9+ CTD (Marine Research Institute); Sea-Bird Electronics 43 oxygen sensor (Marine Research Institute); WET Labs ECO FLNTU, measuring chlorophyll fluorescence with excitation at 470 nm and emission at 695 nm and volume scattering function at 140° and 700 nm (Perry); WET Labs C-Star transmissometer, measuring transmission at 653 nm (s/n 284, loaned to Perry); Biospherical Instruments underwater QSP2300 sensor, measuring scalar underwater photosynthetically active radiation (PAR; Perry). For more details, see sensor set up and instrument calibration factors document: b200804_PsaReport.txt WET Labs C-Star s/n 284 was used on cruise B200804 (deployment cruise on R/S Bjarni Saemundsson). The parameter name beamc_id indicates that C-Star s/n 284 was used for this cruise. Cross calibration of C-Star sensors across different cruises is discussed under Data Processing. Processing Description CTD data are reported as cast number and not as station number. Temperature and salinity (parameter names temp and sal): On the R/S Bjarni Saemundsson cruises, only one CTD unit was present, so salinity and temperature were de-spiked using a 21-point (3.5 s) median filter. An additional 81-point (13 s) median filter was applied where the signal was particularly variable. For more details on temperature and salinity processing, see Ship_TS_despiking-NAB08.pdf. Dissolved oxygen, O2 (parameter name O2_uncal): The sensor was calibrated immediately before the cruise. The factory calibration was applied to SBE43 oxygen sensor voltage, with units of µmol kg-1. Although the sensor was calibrated immediately before the cruise, the absolute concentrations were not verified by Winkler oxygen. Particulate attenuation coefficient, cp (parameter name beam_cp): C-Star s/n 284 did not have a recent factory calibration. On the subsequent R/V Knorr cruise in May, it was cross calibrated with the newly calibrated C-Star s/n 1090 through a series of simultaneous ship/Biofloat48 calibration profiles. The final products for all three C-Stars are cross-calibrated cp coefficients with units of m-1. Complete details of the transmissometer intercalibration procedure are in C-Star calibration report (C-Star_Calibration-NAB08.pdf). Particulate backscattering coefficient, bbp (parameter name bbp700): Backscattering voltage was converted to β at 140° by subtracting dark voltages (median in-situ dark voltage, 0.078 V) and multiplying by factory calibration scale factors, modified based on measurements and calculations of Sullivan et al. (subm.). The calibration factor did not change between pre-cruise and post-cruise factory calibration. β at 140° was converted to bbp (m-1) by subtracting β of seawater (Zhang et al., 2009) and multiplying by 2π (where π = 1.132). See calibration report for more details: Backscatter_Calibration-NAB08.pdf. Chlorophyll fluorescence (parameter name chl_raw) is reported as the raw instrument voltage output minus dark voltage (median in situ dark voltage = 0.083 volts. Water column photosynthetically active radiation (parameter name PAR): The sensor was new and freshly calibrated before the cruise. For more details, see Radiometry_and_PAR_Calibration-NAB08.pdf. Nutrients (parameter names NO3_NO2 and Si_acid): Nutrient samples for nitrate plus nitrite (NO3-+ NO2-) and silicic acid (Si(OH4)) were collected in acid-washed LDPE bottles; unfiltered samples were frozen immediately and stored for up to 8 mo. Samples were thawed at room temperature in the dark for 24 h and vigorously vortexed (Gordon et al., 1993). Samples were analyzed with a Lachat Quickchem 8000 Flow Injection Analysis System using standard absorptiometric techniques (Smith and Bogren, 2001; Wolters, 2002; QuikChem Method 31-107-04-1-C for nitrate plus nitrite; QuikChem Method 31-114-27-1-B for silicic acid). All Lachat spectra were manually inspected for irregularities in baseline or the presence of bubbles. Any offending samples were rejected. Profiles of silicic acid and nitrate concentrations for all casts were also examined, following the recommendation of the IODE workshop on quality control of chemical oceanographic data (IOC, 2010). Concentrations that were clearly out of range without temperature or salinity intrusions were rejected. Phosphate baselines as a whole were not sufficiently stable to be accepted and hence phosphate data are not reported. For additional details on laboratory analysis of discrete water samples see: Laboratory_analysis_report-NAB08.pdf. Fluorometric pigments (parameter names chl_a_fluor and phaeo): Water samples for fluorometric analysis of chlorophyll and pheopigments were filtered through Whatman GF/F filters. Filters were extracted in 5 ml of 90% acetone at -20? C for 24 h and read on a Turner Designs Model 10-AU Digital fluorometer. The fluorometer was calibrated before and after the field program with Turner Designs chlorophyll standards. Chlorophyll and pheopigment were determined following JGOFS protocol procedures (Knap et al., 1996). For additional details on laboratory analysis of discrete water samples see: Laboratory_analysis_report-NAB08.pdf. Particulate organic carbon (parameter name POC): Samples were collected from Niskin bottles using a sampling bell to minimize particle contamination from air. They were immediately filtered, using closed bottles fitted with Biochem Fluidics caps with 2 tubing ports, through a Millipore Swinnex in-line filter holder onto pre-combusted Whatman GF/F filters. All plastics were washed in RBS. Samples were stored frozen for up to 5 mo. Before analysis, samples were dried at 50°C for 4 h, fumed with hydrochloric acid (HCl, 11.65 N) for 12 h, and stored in a desiccator for up to 12 h. Filters were rolled and placed in pre-combusted tin cups shortly before analysis on a Perkin Elmer 2400CHN analyzer (Knap et al., 1996). For additional details on laboratory analysis of discrete water samples see: Laboratory_analysis_report-NAB08.pdf. HPLC pigment samples (see various parameter names) were filtered onto Whatman GF/F filters and preserved in -80°C (liquid nitrogen) until analysis. Samples were stored for up to 5 mo. HPLC analysis was performed by Horn Point laboratories using a methanol-based reversed-phase gradient C8 chromatography column system and appropriate standards (Hooker et al., 2009; Van Heukelem and Thomas, 2001). For additional details on laboratory analysis of discrete water samples see: Laboratory_analysis_report-NAB08.pdf. Particulate and phytoplankton absorption coefficients (parameter names ap(λ) and aph(λ): Water samples for absorption spectra were filtered onto Whatman GF/F filters and scanned at sea on a Varian Cary 50 UV-Visible Spectrophotometer with a xenon flash lamp and a 1.5nm slit width for measurement of total particulate absorption spectra, ap(λ) (Mitchell and Kiefer, 1988). Filters were subsequently extracted in methanol and re-analyzed to determine residual detrital particulate absorption, ad(λ); the difference spectra are reported as phytoplankton absorption spectra, aph(λ) (Kishino et al., 1985). For each sample and blank, the average absorbance between 750-800 nm was subtracted as the zero baseline for that spectrum. For additional details on laboratory analysis of discrete water samples see: Laboratory_analysis_report-NAB08.pdf. |
B9-2008
| Website | |
| Platform | R/V Bjarni Saemundsson |
| Start Date | 2008-06-02 |
| End Date | 2008-06-06 |
| Description | Rescue cruise: R/S Bjarni Saemundsson departed 2 June 2008 2030 from Reykjavik to recover Float 48 and Seaglider 143. Ten CTD casts were made and bottles samples collected for calibration of remaining Seagliders before departing study area. R/S Bjarni Saemundssonreturned to Reykjavik on 6 June 2008. Methods & Sampling CTD rosette deployed from the R/S Bjarni Saemundsson was equipped with following sensors; sensor owner is identified in parenthesis (Marine Research Institute or Perry): Sea-Bird Electronics 11+ CTD deck unit (Marine Research Institute); Sea-Bird Electronics 9+ CTD (Marine Research Institute); Sea-Bird Electronics 43 oxygen sensor (Marine Research Institute); WET Labs ECO FLNTU, measuring chlorophyll fluorescence with excitation at 470 nm and emission at 695 nm and volume scattering function at 140° and 700 nm (Perry); Biospherical Instruments underwater QSP2300 sensor, measuring scalar underwater photosynthetically active radiation (PAR; Perry). For more details, see sensor set up and instrument calibration factors document: b200809_PsaReport.txt Processing Description CTD data are reported as cast number and not as station number. Temperature and salinity (parameter names temp and sal): On the R/S Bjarni Saemundsson cruises, only one CTD unit was present, so salinity and temperature were de-spiked using a 21-point (3.5 s) median filter. An additional 81-point (13 s) median filter was applied where the signal was particularly variable. For more details on temperature and salinity processing, see Ship_TS_despiking-NAB08.pdf. Dissolved oxygen, O2 (parameter name O2_uncal): The sensor was calibrated immediately before the 1 April 2008 cruise. The factory calibration was applied to SBE43 oxygen sensor voltage, with units of µmol kg-1. Although the sensor was calibrated immediately before the cruise, the absolute concentrations were not verified by Winkler oxygen. Particulate backscattering coefficient, bbp (parameter name bbp700): Backscattering voltage was converted to β at 140° by subtracting dark voltages (median in-situ dark voltage, 0.078 V) and multiplying by factory calibration scale factors, modified based on measurements and calculations of Sullivan et al. (subm.). The calibration factor did not change between pre-cruise and post-cruise factory calibration. β at 140° was converted to bbp (m-1) by subtracting β of seawater (Zhang et al., 2009) and multiplying by 2π (where π = 1.132). See calibration report for more details: Backscatter_Calibration-NAB08.pdf. Chlorophyll fluorescence: Chlorophyll fluorescence (parameter name chl_raw) is reported as the raw instrument voltage output minus dark voltage (median in situ dark voltage = 0.083 volts). Water column photosynthetically active radiation (parameter name PAR): The sensor was new and freshly calibrated before the cruise. For more details, see Radiometry_and_PAR_Calibration-NAB08.pdf. |
B10-2008
| Website | |
| Platform | R/V Bjarni Saemundsson |
| Start Date | 2008-06-25 |
| End Date | 2008-07-01 |
| Description | Recovery cruise: R/S Bjarni Saemundsson departed 25 June 2008 0930 from Reykjavik to recover Seagliders 140, 141, 142. Before the ship departed port, SG 142 stopped communicating; hence, a survey pattern was carried out to acoustically ping for the glider but was unsuccessful in locating it. CTD casts were made (n=12) and bottles samples collected for calibration of SG 140 and 141 before they were recovered. The ship steamed to near the original deployment site (59.02º, -20.49) on 29 June 2008 to deploy two bio-optical ARGO floats for Dr. H. Claustre, LOV, France. R/S Bjarni Saemundsson returned to Reykjavik on 1 July 2008.
Methods & Sampling CTD rosette deployed from the R/S Bjarni Saemundsson was equipped with following sensors. Sensor owner is identified in parenthesis (Marine Research Institute or Perry): Sea-Bird Electronics 11+ CTD deck unit (Marine Research Institute); Sea-Bird Electronics 9+ CTD (Marine Research Institute); Sea-Bird Electronics 43 oxygen sensor (Marine Research Institute); WET Labs ECO FLNTU, measuring chlorophyll fluorescence with excitation at 470 nm and emission at 695 nm and volume scattering function at 140° and 700 nm (Perry); WET Labs C-Star transmissometer, measuring transmission at 653 nm (s/n 284, loaned to Perry); Biospherical Instruments underwater QSP2300 sensor, measuring scalar underwater photosynthetically active radiation (PAR; Perry). For more details, see sensor set up and instrument calibration factors document (b200810_PsaReport.txt) contributed by original investigators. Processing Description CTD data are reported as cast number and not as station number. Temperature and salinity (parameter names temp and sal): On the R/S Bjarni Saemundsson cruises, only one CTD unit was present, so salinity and temperature were de-spiked using a 21-point (3.5 s) median filter. An additional 81-point (13 s) median filter was applied where the signal was particularly variable. For more details on temperature and salinity processing, see Ship_TS_despiking-NAB08.pdf. Dissolved oxygen, O2 (parameter name O2_uncal): The sensor was calibrated immediately before the 1 April cruise. The factory calibration was applied to SBE43 oxygen sensor voltage, with units of µmol kg-1. Although the sensor was calibrated immediately before the cruise, the absolute concentrations were not verified by Winkler oxygen. Particulate attenuation coefficient, cp (parameter name beam_cp): The factory calibration was used to convert C-Star s/n 1090 voltage to particulate attenuation coefficient, cp, (m-1); the calibration factor did not change between pre-cruise and post-cruise factory calibration. C-Star s/n 284 was cross calibrated with C-Star s/n 1090 through a series of simultaneous ship/Biofloat48 calibration profiles. The final product contains cross-calibrated cp coefficients for all three C-Stars. Complete details of the transmissometer intercalibration procedure are in C-Star calibration report (C-Star_Calibration-NAB08.pdf). Particulate backscattering coefficient, bbp (parameter name bbp700): Backscattering voltage was converted to β at 140° by subtracting dark voltages (median in-situ dark voltage, 0.078 V) and multiplying by factory calibration scale factors, modified based on measurements and calculations of Sullivan et al. (subm.). The calibration factor did not change between pre-cruise and post-cruise factory calibration. β at 140° was converted to bbp (m-1) by subtracting β of seawater (Zhang et al., 2009) and multiplying by 2 π(where π = 1.132). See calibration report for calibration report for more details: Backscatter_Calibration-NAB08.pdf. Chlorophyll fluorescence: Chlorophyll fluorescence (parameter name chl_raw) is reported as the raw instrument voltage output minus dark voltage (median in situ dark voltage = 0.083 volts). Water column photosynthetically active radiation (parameter name PAR): The sensor was new and freshly calibrated before the cruise. For more details, see Radiometry_and_PAR_Calibration-NAB08.pdf. Nutrients (parameter names NO3_NO2 and Si_acid): Nutrient samples for nitrate plus nitrite (NO3-+ NO2-) and silicic acid (Si(OH4)) were collected in acid-washed LDPE bottles; unfiltered samples were frozen immediately and stored for up to 8 mo. Samples were thawed at room temperature in the dark for 24 h and vigorously vortexed (Gordon et al., 1993). Samples were analyzed with a Lachat Quickchem 8000 Flow Injection Analysis System using standard absorptiometric techniques (Smith and Bogren, 2001; Wolters, 2002; QuikChem Method 31-107-04-1-C for nitrate plus nitrite; QuikChem Method 31-114-27-1-B for silicic acid). All Lachat spectra were manually inspected for irregularities in baseline or the presence of bubbles. Any offending samples were rejected. Profiles of silicic acid and nitrate concentrations for all casts were also examined, following the recommendation of the IODE workshop on quality control of chemical oceanographic data (IOC, 2010). Concentrations that were clearly out of range without temperature or salinity intrusions were rejectedA total of 14 samples were rejected for nitrate and 11 for silicate out of 91 total samples. Phosphate baselines as a whole were not sufficiently stable to be accepted and hence phosphate data are not reported. For additional details on laboratory analysis of discrete water samples see: Laboratory_analysis_report-NAB08.pdf. Fluorometric pigments (parameter names chl_a_fluor and phaeo): Water samples for fluorometric analysis of chlorophyll and pheopigments were filtered through Whatman GF/F filters. Filters were extracted in 5 ml of 90% acetone at -20° C for 24 h and read on a Turner Designs Model 10-AU Digital fluorometer. The fluorometer was calibrated before and after the field program with Turner Designs chlorophyll standards. Chlorophyll and pheopigment were determined following JGOFS protocol procedures (Knap et al., 1996). For additional details on laboratory analysis of discrete water samples see: Laboratory_analysis_report-NAB08.pdf. Particulate organic carbon (parameter name POC): Samples were collected from Niskin bottles using a sampling bell to minimize particle contamination from air. They were immediately filtered, using closed bottles fitted with Biochem Fluidics caps with 2 tubing ports, through a Millipore Swinnex in-line filter holder onto pre-combusted Whatman GF/F filters. All plastics were washed in RBS. Samples were stored frozen for up to 5 mo. Before analysis, samples were dried at 50 °C for 4 h, fumed with hydrochloric acid (HCl, 11.65 N) for 12 h, and stored in a desiccator for up to 12 h. Filters were rolled and placed in pre-combusted tin cups shortly before analysis on a Perkin Elmer 2400CHN analyzer (Knap et al., 1996). For additional details on laboratory analysis of discrete water samples see: Laboratory_analysis_report-NAB08.pdf. HPLC pigment samples (see various parameter names) were filtered onto Whatman GF/F filters and preserved in -80 °C (liquid nitrogen) until analysis. Samples were stored for up to 5 mo. HPLC analysis was performed by Horn Point laboratories using a methanol-based reversed-phase gradient C8 chromatography column system and appropriate standards (Hooker et al., 2009; Van Heukelem and Thomas, 2001). For additional details on laboratory analysis of discrete water samples see: Laboratory_analysis_report-NAB08.pdf. Particulate and phytoplankton absorption coefficients (parameter names ap(?) and aph(?): Water samples for absorption spectra were filtered onto Whatman GF/F filters and scanned at sea on a Varian Cary 50 UV-Visible Spectrophotometer with a xenon flash lamp and a 1.5nm slit width for measurement of total particulate absorption spectra, ap(?) (Mitchell and Kiefer, 1988). Filters were subsequently extracted in methanol and re-analyzed to determine residual detrital particulate absorption, ad(?); the difference spectra are reported as phytoplankton absorption spectra, aph(?) (Kishino et al., 1985). For additional details on laboratory analysis of discrete water samples see: Laboratory_analysis_report-NAB08.pdf. |
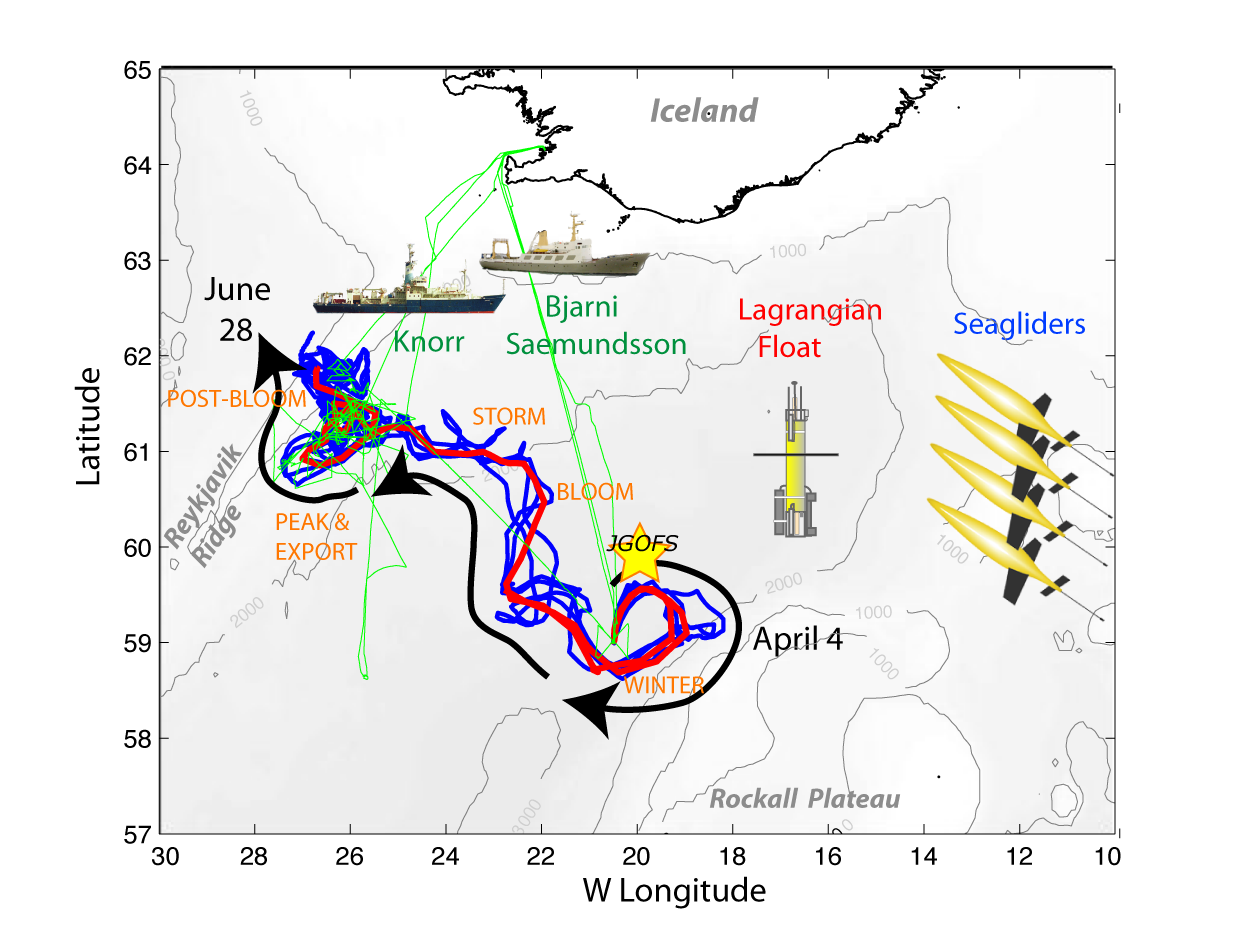
North Atlantic Bloom Experiment 2008 (NAB 2008)
NAB2008 was a process experiment designed to study an important component of the oceanic carbon system - the North Atlantic spring bloom. The phytoplankton bloom occurring each spring in the North Atlantic, drives the uptake of carbon dioxide and is an important component of the biological pump (Bagniewski et al., 2010). Previous studies in this region have shown the importance of small temporal and spatial scales, i.e. ecosystem patchiness, during the bloom, but were restricted by the limitations of ship-based sampling. Recent advances in autonomous platforms and sensors presented an opportunity to study this important event in a new way. In addition to deployment of a diverse suite of in situ sampling devices, NAB2008 was also a test-bed for developing the strategies and knowledge needed to successfully use new methods to drive the next generation of ocean observations.
In 2008, a coordinated deployment of 1 float, 4 Seagliders and 2 research vessels sampled the evolution of the North Atlantic spring bloom along and surrounding the nearly Lagrangian path followed by the float. The autonomous measurements were continuous through the experimental period, and included CTD, chlorophyll fluorescence, optical backscatter, and oxygen on all platforms; and nitrate, optical attenuation, and various radiance measurements on the float. Velocities were determined from the vehicle motion, with the float extending to a depth of 230 meters and gliders to 1,000 meters. The autonomous vehicles were deployed, rescued, and recovered on three cruises of the Icelandic vessel Bjarni Saemundsson. A 21-day cruise of the R/V Knorr conducted more detailed measurements during the peak of the bloom in May. The R/V Knorr sampling program included optical profiles, ADCP data and analysis of water samples for nutrients, particulate organic carbon, pigments, micro-plankton composition, complemented by guest investigator analyses. Data from both ships were used to calibrate and validate the autonomous measurements.
References:
Bagniewski, W., Fennel, K., Perry, M. J., and D'Asaro, E. A. (2010) Optimizing models of the North Atlantic spring bloom using physical, chemical and bio-optical observations from a Lagrangian float, Biogeosciences Discuss., 7, pp. 8477-8520, doi:10.5194/bgd-7-8477-2010
Ocean Carbon and Biogeochemistry (OCB)
The Ocean Carbon and Biogeochemistry (OCB) program focuses on the ocean's role as a component of the global Earth system, bringing together research in geochemistry, ocean physics, and ecology that inform on and advance our understanding of ocean biogeochemistry. The overall program goals are to promote, plan, and coordinate collaborative, multidisciplinary research opportunities within the U.S. research community and with international partners. Important OCB-related activities currently include: the Ocean Carbon and Climate Change (OCCC) and the North American Carbon Program (NACP); U.S. contributions to IMBER, SOLAS, CARBOOCEAN; and numerous U.S. single-investigator and medium-size research projects funded by U.S. federal agencies including NASA, NOAA, and NSF.
The scientific mission of OCB is to study the evolving role of the ocean in the global carbon cycle, in the face of environmental variability and change through studies of marine biogeochemical cycles and associated ecosystems.
The overarching OCB science themes include improved understanding and prediction of: 1) oceanic uptake and release of atmospheric CO2 and other greenhouse gases and 2) environmental sensitivities of biogeochemical cycles, marine ecosystems, and interactions between the two.
The OCB Research Priorities (updated January 2012) include: ocean acidification; terrestrial/coastal carbon fluxes and exchanges; climate sensitivities of and change in ecosystem structure and associated impacts on biogeochemical cycles; mesopelagic ecological and biogeochemical interactions; benthic-pelagic feedbacks on biogeochemical cycles; ocean carbon uptake and storage; and expanding low-oxygen conditions in the coastal and open oceans.
| Funding Source | Award |
|---|---|
| NSF Division of Ocean Sciences (NSF OCE) | |
| National Aeronautics & Space Administration (NASA) | |
| NSF Division of Ocean Sciences (NSF OCE) |
[ table of contents | back to top ]