Depth profiles of dissolved, small and large particulate 232Th, 230Th, and 231Pa from R/V Knorr KN199-04, KN204-01, subtropical North Atlantic Ocean from 2010-2011 (U.S. GEOTRACES NAT project)
Project
Program
| Contributors | Affiliation | Role |
|---|---|---|
| Anderson, Robert F. | Lamont-Doherty Earth Observatory (LDEO) | Lead Principal Investigator |
| Edwards, R. Lawrence | University of Minnesota Twin Cities (UMTC) | Principal Investigator |
| Robinson, Laura F. | Woods Hole Oceanographic Institution (WHOI BCO-DMO) | Principal Investigator |
| Cheng, Hai | University of Minnesota Twin Cities (UMTC) | Co-Principal Investigator |
| Fleisher, Martin Q. | Lamont-Doherty Earth Observatory (LDEO) | Co-Principal Investigator |
| Hayes, Christopher T. | Lamont-Doherty Earth Observatory (LDEO) | Contact |
| Huang, Kuo-Fang Denner | Woods Hole Oceanographic Institution (WHOI) | Contact |
| Lu, Yanbin | University of Minnesota Twin Cities (UMTC) | Contact |
| Copley, Nancy | Woods Hole Oceanographic Institution (WHOI BCO-DMO) | BCO-DMO Data Manager |
DMO NOTE:
- Version 4b: 16 May 2016; metadata text revised.
- Version 4: 29 Sept 2015; combined both dissolved and particulate Th & Pa datasets into one.
- Version 3: 17 Mar 2014; revised dataset
- Version 2: 7 Nov. 2013
- Version 1: 7 Jan 2013
Sampling Methodology:
Dissolved data:
Water samples were collected with a Sea-Bird Electronics CTD carousel fitted with
12 30-liter PVC Niskin bottles, maintained and operated by the Ocean Data Facility of Scripps Institution of Oceangraphy. The carousel was lowered from the ship with steel wire. Niskin bottles were equipped with nylon-coated closure springs and Viton O-rings. After collection seawater was drained with Teflon-lined TygonTM tubing and filtered through Pall AcropakTM 500 filters on deck (gravity filtration, 0.8/0.45 μm pore size) into Fisher I-Chem series 300 LDPE cubitainers. Approximately 4-5 L was collected per desired depth. Prior to the cruise, the tubing, filters and cubitainers were cleaned by immersion in 1.2 M HCl (Fisher Scientific Trace Metal Grade) for 4-5 days. Once filtered, samples were adjusted to a pH ~2 with ultra-clean 6 M HCl (Fisher Scientific OPTIMA grade), double-bagged, stored in pallet boxes on-deck until the end of the cruise and then at room temperature once shipped to the participating laboratories for analysis.
Particulate data:
Size-fractionated particles were collected using McLane Research in-situ pumps (WTS-LV) that had been modified to accommodate two flowpaths (Lam and Morris Patent pending). The wire-out was used to target depths during deployment, and a self-recording Seabird 19plus CTD deployed at the end of the line and RBR data loggers attached to three of the eight pumps were used to correct for actual depths during pumping.
Filter holders used were 142 mm-diameter “mini-MULVFS” style filter holders with two stages for two size fractions and multiple baffle systems designed to ensure even particle distribution and prevent particle loss (Bishop et al. 2012). One of two filter holder/flowpaths was loaded with a 51µm Sefar polyester mesh prefilter followed by paired 0.8 µm Pall Supor800 polyethersulfone filters. Each cast also had “dipped blank” filters deployed. These were the full filters sets (prefilter followed by paired Supor filters) sandwiched within a 1 µm polyester mesh filter, loaded into perforated polypropylene containers, attached with plastic cable ties to a pump frame, and deployed. Dipped blank filters were exposed to seawater for the length of the deployment and processed and analyzed as regular samples, and thus functioned as full seawater process blanks. We analyzed half portions of the top and bottom filters from the “dipped” blank from 1 or more depths for 7 stations.
All filters and filter holders were acid leached prior to use according to methods recommended in the GEOTRACES sample and sample-handling Protocols (Geotraces 2010).
Analytical methods for dissolved radionuclides:
LDEO:
In the on-shore laboratory, samples were weighed to determine sample size, taking into account the weight of the cubitainer and of the acid added at sea. Then weighed aliquots of the artificial isotope yield monitors 229Th (20 pg) and 233Pa (0.5 pg) and 15 mg dissolved Fe were added to each sample. After allowing 1 day for spike equilibration, the pH of each sample was raised to 8-8.5 by adding ~10 mL of concentrated NH4OH (Fisher Scientific OPTIMA grade) which caused iron (oxy)hydroxide precipitates to form. This precipitate was allowed to settle for 1-2 days before the overlaying seawater was siphoned off. The Fe precipitate was transferred to centrifuge tubes for centrifugation and rinsing with Milli-Q H2O (>18 MΩ) to remove the major seawater ions. The precipitate was then dissolved in 16 M HNO3 (Fisher Scientific OPTIMA grade) and transferred to a Teflon beaker for a high-temperature (180-200°C) digestion with HClO4 and HF (Fisher Scientific OPTIMA grade) on a hotplate in a HEPA-filtered laminar flow hood. After total dissolution of the sample, another precipitation of iron (oxy)hydroxide followed and the precipitate was washed with Mill-Q H2O, centrifuged, and dissolved in 12 M HCl for a series of anion-exchange chromatography using 6 mL polypropylene columns each containing a 1 mL bed of Bio-rad resin (AG1-X8, 100-200 mesh size) and a 45 μm porous polyethylene frit (Anderson et al. 2012). The final column elutions were dried down at 180°C in the presence of 2 drops of HClO4 and taken up in approximately 1 mL of 0.16 M HNO3/0.026 M HF for mass spectrometric analysis.
Concentrations of 232Th, 230Th and 231Pa were calculated by isotope dilution using nuclide ratios determined on a VG Elemental AXIOM Single Collector Magnetic Sector ICP-MS with a Resolving Power of ~400 to ensure the highest sensitivity. All measurements were done using a peak jumping routine in ion counting mode. A solution of SRM129, a natural U standard, was run to determine the mass bias correction (assuming that the mass fractionation for Th and Pa are the same as for U). Each sample measurement was bracketed by measurement of an aliquot of the run solution, used to correct for the instrument background count rates on the masses measured. To correct for potential tailing of 232Th into the minor Th and Pa isotopes, beam intensities were measured at the half masses above and below each mass for 230Th, 231Pa, and 233Pa. Tailing under each minor isotope was estimated as the log mean intensity of the half masses on either side of each minor isotope.
Water samples were analyzed in batches of 10-12. Procedural blanks were determined by processing 4-5 L of Milli-Q water in an acid-cleaned cubitainer acidified to pH ~2 with 6 M HCl as a sample in each batch. An aliquot of an intercalibrated working standard solution of 232Th, 230Th and 231Pa, SW STD 2010-1 referred to by Anderson et al. (2012), was added to a separate cubitainer with 5 L of Milli-Q water (acidified to pH 2) and also processed like a sample in each batch. Total procedural blanks for 232Th, 230Th, and 231Pa ranged from 4.2-20.9 pg, 0.8-2.2 fg, and 0-0.85 fg, respectively.
UMN:
In the on-shore laboratory, samples were weighed to determine sample size, taking into account the weight of the cubitainer and of the acid added at sea. Then weighed aliquots of the artificial isotope yield monitors 229Th and 233Pa and several milligrams dissolved Fe were added to each sample. After allowing 3 day for spike equilibration (at a temperature of about 40oC, the pH of each sample was raised to 8-8.5 by adding concentrated NH4OH which caused iron (oxy)hydroxide precipitates to form. This precipitate was allowed to settle for 1-2 days before the overlaying seawater was siphoned off. The Fe precipitate was transferred to centrifuge tubes for centrifugation and rinsing with deionized H2O (>18 MΩ) to remove the major seawater ions. The precipitate was then dissolved in 14 M HNO3 and transferred to a Teflon beaker. It was then dried down and taken up in 7N HNO3 for anion-exchange chromatography using AG1-X8, 100-200 mesh size resin and polyethylene frit. Initial separation was done on Teflon columns with a 0.75 ml column volume (CV). The sample was loaded in one CV of 7N HNO3, followed by 1.5 CV of 7N HNO3, 3 CV of 8N HCl (collect Th fraction), and 3 CV of 8N HCl combined with 0.015N HF (collect Pa fraction). The Pa and Th fractions were then dried down and taken up in 7N HNO3. They were each passed through second and third columns (each with 0.5 ml column volumes) using similar elution schemes. The final Pa and Th fractions were then dried down and dissolved in weak nitric acid for analysis on the mass spectrometer.
Concentrations of 232Th, 230Th and 231Pa were calculated by isotope dilution using nuclide ratios determined on a Thermo-Finnigan Neptune mass spectrometer. All measurements were done using a peak jumping routine in ion counting mode on the discreet dynode multiplier behind the retarding potential quadrupole. A solution of 233U-236U tracer was run to determine the mass bias correction (assuming that the mass fractionation for Th and Pa are the same as for U). Each sample measurement was bracketed by measurement of an aliquot of the run solution used to correct for the instrument background count rates on the masses measured.
Procedural blanks for chemical and mass spectrometric analyses at Minnesota are about 700 fg for 232Th, 15 ag for 230Th, and 20 ag for 231Pa.
Further details on Pa and Th analysis at the U. Minnesota laboratory are given in Shen et al. (2002, 2003, 2012) and Cheng et al. (2000).
WHOI:
In the on-shore laboratory, the analytical procedures followed the protocols described in Auro et al. (2012). Briefly, samples were weighed to determine sample size, taking into account the weight of the cubitainer. Then weighed aliquots of the artificial isotope yield monitors 229Th and 233Pa and ~100 mg dissolved Fe were added to each sample. After allowing 1 day for spike equilibration, the pH of each sample was raised to 7.5-8 by adding ~10 mL of concentrated NH4OH (Fisher Scientific OPTIMA grade) which caused iron (oxy)hydroxide precipitates to form. This precipitate was allowed to settle for 5-7 days before the overlaying water was siphoned off. The Fe precipitate and remaining water was transferred to polypropylene centrifuge tubes for centrifugation and rinsing with pH 8 Milli-Q H2O (>18 MΩ) to remove the major seawater ions. The precipitate was then transferred into Teflon centrifuge tubes, re-rinsed and dissolved in 12 M HCl (Fisher Scientific OPTIMA grade) for a series of anion-exchange chromatography using 10 mL polypropylene columns each containing a 0.5 mL bed of Eichrom Technologies pre-filter resin in addition to 5 mL Anion Exchange Resin (1x8, 100-200 mesh; Eichrom Technologies) for Th elution and 1.5 mL Anion Exchange Resin for Pa. A 236U tracer was added to the Th fraction to assist in monitoring signal intensity changes during mass spectrometry. The final column elutions were dried down at 150°C in the presence of 1 mL of 16 M HNO3 (Fisher Scienntific OPTIMA grade) and taken up in 1 mL of 0.8 M HNO3/0.13 M HF for mass spectrometric analysis.
Concentrations of 232Th, 230Th and 231Pa were calculated by isotope dilution using nuclide ratios determined on a Neptune Multi Collector ICP-MS (Auro et al. 2012). Thorium isotopes were measured using a peak jumping routine with 229Th and 230Th analyzed on the central Secondary Electron Multiplier (SEM) and 232Th and 236U measured concurrently on Faraday collectors. Mass bias and ion counter yields were corrected for using an in-house thorium standard. Peak tails were calculated from the 232Th intensity using the pre-determined size of the tail at 2 and 3 amu for 230Th and 229Th respectively. Accuracy was assessed using a secondary consistency standard made at WHOI. 231Pa and 233Pa were analyzed simultaneously on ion counting channels. A solution of CRM145, a natural U standard, was run to determine the mass bias (assuming that the mass fractionation for Th and Pa are the same as for U) and ion counter yields. Sample measurements were bracketed by measurement of an aliquot of the run solution, used to correct for the instrument background count rates on the masses measured. To correct for potential tailing onto the Pa isotopes, beam intensities were measured at the half masses.
Water samples were analyzed in batches of 10-12. Procedural blanks were determined by processing 4-5 L of Milli-Q water in an acid-cleaned cubitainer acidified to pH ~2 with 12 M HCl as a sample in each batch. One or two aliquots of an intercalibrated working standard solution of 232Th, 230Th and 231Pa, SW STD 2010-1 referred to by Anderson et al. (2012), was added to a separate cubitainer with 5 L of Milli-Q water (acidified to pH 2) and also processed like a sample with each batch. Total procedural blanks for 232Th, 230Th, and 231Pa (with the exception of one batch described below) ranged from 3-15 pg, 0.1- 0.9 fg, and 0.1-1.1 fg respectively. One batch had an anomalously high 232Th blank of >2500pg (with 230Th and 231Pa of 4.4 and 0.3 fg respectively) which was found to be due to a batch of contaminated acid. The 232Th concentrations for that batch are not reported.
Further details on sampling and analysis are given by Anderson et al. (2012).
Analytical Methods for particulate radionuclides:
The Supor filters were subsampled in an on-shore laboratory at the Woods Hole Oceanographic Institution and shipped to the participating labs for Pa/Th analysis. Twenty-five to 50% of the paired Supor filters, representing 55-350 L of seawater, were used for Pa/Th analysis. Analyses were similar but differed slightly for the Lamont-Doherty Earth Observatory, WHOI and the University of Minnesota. Details of each groups methodologies can be found in reports by Anderson et al. (2012), Auro et al. (2012) and Shen et al. (2002, 2003, 2012), respectively. Below we give a typical procedure used at LDEO for illustrative purposes.
LDEO procedures:
Filters were folded into 60 mL Teflon jars and weighed aliquots of the artificial isotope yield monitors 229Th (1 pg) and 233Pa (0.3-0.4 pg) and 7-8 mg dissolved Fe were added to each sample. Filters were first heated in ~5 mL 8 N HNO3 for 1-2 hours at 150°C, then 4-5 mL HClO4 was added and heat was increased to 200°C until dense white fumes appeared for ~10-20 min. The heat was then reduced to 180°C and the samples were covered with a Teflon watch cover. After 1-4 hrs, oxidation of the Supor material accelerated, sometimes producing a foam. A foamed sample would be allowed to cool, and re-heated after the beaker walls and watch cover were washed with small amounts of HNO3 or Milli-Q water. When the Supor material was largely broken down, the watch covers were removed and HF was added in 2 aliquots of ~10-15 drops in between reheating until attaining dense HClO4 fumes for at least 10 min.
After total dissolution of the sample, the sample-HClO4 residue was taken up in dilute HCl, and transferred to 50 mL centrifuge tubes with water rinses. Ten to 20 drops of NH4OH were added to raise pH to 8-8.5 when iron (oxy)hydroxide precipitated. This precipitate was then centrifuged, decanted, washed with Milli-Q H2O, centrifuged, and dissolved in 12 M HCl for a series of anion-exchange chromatography using 6 mL polypropylene columns each containing a 1 mL bed of Bio-rad resin (AG1-X8, 100-200 mesh size) and a 45 μm porous polyethylene frit (Anderson et al. 2012). The final column elutions were dried down at 180°C in the presence of 2 drops of HClO4 and taken up in approximately 1 mL of 0.16 M HNO3/0.026 M HF for mass spectrometric analysis. All acids and bases used were Fisher Chemical OPTIMA grade.
Concentrations of 232Th, 230Th and 231Pa were calculated by isotope dilution using nuclide ratios determined on a Thermo Scientific Element XR Inductively-couple plasma mass spectrometer (ICP-MS) in low resolution. All measurements were done using a peak jumping routine in ion counting mode. A solution of SRM129, a natural U standard, was run to determine the mass bias correction (assuming that the mass fractionation for Th and Pa are the same as for U). Each sample measurement was bracketed by measurement of an aliquot of the run solution, used to correct for the instrument background count rates on the masses measured. To correct for potential tailing of 232Th into the minor Th and Pa isotopes, beam intensities were measured at the half masses above and below each mass for 230Th, 231Pa, and 233Pa. Tailing under each minor isotope was estimated as the log mean intensity of the half masses on either side of each minor isotope.
In addition to laboratory procedural blanks (reagents/labware blanks) and periodic measurements of an intercalibrated working standard solution of 232Th, 230Th and 231Pa, SW STD 2010-1 referred to by Anderson et al. (2012), the participating labs also analyzed a number (n = 23) of “dipped blank” filters, mentioned above, to determine the total blank, associated with the sample collection and handling in addition to the laboratory procedure.
For better statistics, we pooled all procedural blank corrected “dipped” blanks (n = 23) to determine filter blank corrections. “Dipped” filter blanks for 232Th, 230Th, and 231Pa were from 156 ± 57 pg, 5.8 ± 2.0 fg, and 0.12 ± 0.04 fg, respectively. Total blanks were < 10% of the measured isotope amounts, except shallower than 200 m water depth, where blanks could be on the order of 50% of the measured 230Th and 231Pa.
We define the limit of detection as 3 times the standard deviation in the measured “dipped” blanks (170 pg 232Th, 6.0 fg 230Th, and 0.13 fg 231Pa). There were 5 samples for which 231Pa was considered below detection, and all other samples were above the cited limits.
Further details on analysis of seawater particulate radionuclides are given by Anderson et al. (2012).
UMN Procedures:
Filters were folded into 30 mL Teflon beaker and weighed aliquots of the artificial isotope yield monitors 229Th and 233Pa were added. Filters were first completely submerged in 7N HNO3 acid combined with 10 drops HF, tightly covered with a Teflon threaded cap and heated for 10 hours at 200°F so that the particulate sample was dissolved/leached under pressure. The leach solution was then transferred to a second acid-cleaned Teflon beaker separate from the residual filter. 5 drops of HClO4 were then added to the leach solution in the second beaker. The original beaker walls and caps were washed with small amounts of weak HNO3 and the resulting solution added to the second beaker. The solution was then dried down and was taken up in 2N HCl, and transferred to 15ml centrifuge tubes along with a 2N HCl rinse. One drop of dissolved Fe and six to nine drops of NH4OH were added to raise pH to 8-8.5 at which time iron (oxy)hydroxide precipitated. This precipitate was then centrifuged, decanted, washed with deionized H2O (>18 MΩ), centrifuged, and dissolved in 14M HNO3 and transferred to a Teflon beaker. It was then dried down and taken up in 7N HNO3 for anion-exchange chromatography using AG1-X8, 100-200 mesh resin and a polyethylene frit. Initial separation was done on Teflon columns (internal diameter ~ 0.35cm) with a ~0.55 ml column volume (CV). The sample was loaded in one CV of 7N HNO3, followed by 1.5 CV of 7N HNO3, 3 CV of 8N HCl (collect Th fraction), and 3 CV of 8N HCl combined with 0.015N HF (collect Pa fraction). The Pa and Th fractions were then dried down in the presence of 2 drops of HClO4 and taken up in 7N HNO3. They were each passed through second and third columns (each with ~0.55 ml column volumes) using similar elution schemes. The final Pa and Th fractions were then dried down in the presence of 2 drops of HClO4 and dissolved in weak nitric acid for analysis on the mass spectrometer.
Concentrations of 232Th, 230Th and 231Pa were calculated by isotope dilution using nuclide ratios determined on a Thermo-Finnigan Neptune mass spectrometer. All measurements were done using a peak jumping routine in ion counting mode on the discreet dynode multiplier behind the retarding potential quadrupole. A solution of 233U-236U tracer was run to determine the mass bias correction (assuming that the mass fractionation for Th and Pa are the same as for U). Each sample measurement was bracketed by measurement of an aliquot of a wash solution, used to correct for the instrument background count rates on the masses measured.
Particulate samples were analyzed in batches of 37 to 39. An aliquot of an intercalibrated working standard solution of 232Th, 230Th and 231Pa, SW STD 2010-1, was added to a separate acid-cleaned Teflon beaker along with weighed aliquots of 229Th spike and 233Pa spike. Spike and Standard were equilibrated for 3 days. The solution was then dried down and taken up in 7N HNO3 for anion-exchange chromatography using AG1-X8, 100-200 mesh resin and a polyethylene frit, and processed like a sample. In addition to laboratory procedural blanks (reagents/labware blanks), a number of “dipped blank” filters were also processed like samples, to determine the total blank, associated with the sample collection and handling, in addition to the laboratory procedure.
WHOI procedures (Microwave-assisted acid digestion):
Sample digestions were accomplished by microwave-assisted acid digestion performed in a Multiwave 3000 (Anton Paar GmbH, Graz, Austria) instrument. Each sample (1/2 filter) were cut into two quarter filters, and put into two pre-cleaned microwave PFA vessels with 12 mL of Aqua Regia (HCl : HNO3= 3:1) and 0.3 mL of HF for the sample digestion. The microwave procedure consisted of three main steps: (1) a 20 min power increasing ramp; (2) the power was electronically adjusted to maintain the internal temperature (600W) at 200°C for 90 min, and (3) a 20 min power decreasing ramp. During the session, the temperature ranged from 190°C and 230°C from one vessel to the other.
The two digested quarter filters were combined into one full sample (1/2 filter). Each sample was transferred to 50mL teflon centrifuge tubes with milliQ water rinses. Purified Fe was added to each sample, which was then spiked with 229Th, 233Pa and 236U (Auro et al 2012), and allowed to equilibrate for at least one day. Ten to 20 drops of NH4OH were added to raise pH to 7.5-8.0 when iron (oxy)hydroxide precipitated. This precipitate was centrifuged, the supernatant decanted, and the remaining precipitate rinsed with pH 8 Milli-Q H2O, centrifuged, and dissolved in concentrated HCl. The sample was purified and separated using a series of anion-exchange chromatography steps (Auro et al 2012) using 7 mL polypropylene columns each containing Eichrom resin (AG1-X8, 200-400 mesh) and Eichrom pre-filter resin (100-150 μm). The final column elutions were dried down at 180°C and re-dissolved in one drop of concentrated HNO3. Dried down and taken up in approximately 1 mL of 5% HNO3+0.13N HF and 1 drop of concentrated HNO3 for mass spectrometric analysis. All acids and bases used were Fisher Chemical OPTIMA grade.
Concentrations of 232Th, 230Th and 231Pa were calculated by isotope dilution (229Th-233Pa spikes) using isotopic ratios determined on a Thermo Scientific Neptune Multi-Collector Inductively-couple plasma mass spectrometer (MC-ICP-MS) coupled to Cetac Aridus I in Low-Resolution mode (Auro et al 2012). For the Th isotopes, measurements on 229Th and 230Th were done using a “peak-hopping” method where 229Th and 230Th were each analyzed on the central SEM. The 232Th beam was analyzed in both steps on Faraday Cup, allowing direct determinations of 232Th/230Th and 232Th/229Th. Mass bias correction was assessed using the uranium standard of CRM-145 (assuming that the mass fractionation for Th and Pa are the same as for U), and the accuracy of the method was evaluated by two in-house standards (ThSGS and ThB, Robinson et al., 2005; Auro et al., 2012). For the Pa isotopes, 231Pa and 233Pa were analyzed on the Multi-Ion Counts (MICs) simultaneously. The yield for each ion counters was checked using the mass bias corrected 234U/238U ratio of CRM-145 uranium standard. To correct for potential tailing of 232Th into the minor Th and Pa isotopes, beam intensities were measured at the half masses above and below each mass for 230Th, 231Pa, and 233Pa. Tailing under each minor isotope was estimated as the log mean intensity of the half masses on either side of each minor isotope.
Parameter names, definitions and units notes:
Radionuclide concentrations are given as micro-Becquerel (10-6 Bq, µBq or micro-Bq) per kg seawater for 230Th and 231Pa, and pmol (10-12 mol) per kg seawater for 232Th. A Becquerel is the SI unit for radioactivity and is defined as 1 disintegration per second. These units are recommended by the GEOTRACES community.
“Dissolved” (D) here refers to that which passed through a 0.45 µm AcropakTM 500 filter capsule sampled from conventional Niskin bottles. This is true for all dissolved samples except for a select number that came from a towed pumping system designed to collect uncontaminated water at 2-3 m depth, indicated by FISH or Stn-GeoF in the sample bottle type. Surface Fish samples were filtered by a 0.2 µm Osmonics filter capsule, unlike Niskin samples.
The “small particulate” (SP) data refers to the particle size class 0.8-51 µm and is sometimes also called the “suspended” size fraction. The “large particulate” (LP) data refers to particles greater than 51 µm and sometimes referred to as the “sinking” size fraction. The particulate samples were collected by in-situ pumping over paired 0.8mm Pall Supor800 polyethersulfone filters behind a 51 mm Sefar polyester mesh prefilter (See Lam et al. 2015 for particulate sampling methodology). Analysis of the paired Supor filters represents a particle size class approximating 0.45-51 µm (Bishop et al. 2012), while the top filter alone represents 0.8-51 µm and it is this size class referred to here as the small particulate fraction. We measured a select number of top and bottom filters separately for radionuclides and found that the bottom filters had radionuclide levels that were indistinguishable from clean filter process blanks. Therefore whether or not samples were analyzed as top and bottom paired, or the top filter alone, we infer the small particulate data to represent 0.8-51 µm particles. The large particulate data is based on analysis of the 51 µm Sefar polyester mesh prefilter. Only a small selection of large particle samples (16) were analyzed at the University of Minnesota.
For dissolved, seawater was weighed directly in the laboratory to determine sample size, taking into account acid added at sea. For particulates, sample size was measured by volume (liters) of seawater pumped by mass flow controllers on the in-situ pumps, but we converted seawater volume to seawater mass using a fixed seawater density of 1.025 kg/L. Concentrations below detection are listed as “bdl”. The abbreviation “nd” refers to no data available.
Parameter names in the form such as “Th_232_D_CONC_BOTTLE” are adopted based on a recommendation from the GEOTRACES community (http://www.egeotraces.org/Parameter_Naming_Conventions.html).
This is compiled data produced by three laboratories with the following associations: Lamont-Doherty Earth Observatory of Columbia University (LDEO), Woods Hole Oceanographic Institution (WHOI) and the University of Minnesota (UMN).
The primary reference for the dissolved data is Hayes et al. (2015, Deep-Sea Research Part II) and for the particulate data, Hayes et al. (2015, Marine Chemistry).
See also Notes on Derived Parameters (pdf), which include those containing 'XS' or 'ADS' in their name.
The reported errors for radionuclide concentrations represent the propagation of one sigma errors based on the standard isotope ratios collected by ICP-MS, estimated error in the 229Th or 233Pa spike concentration, and the blank correction of the individual isotopes for each sample batch.
Analysis of all samples was completed over the course of several years. A correction was made to account for the ingrowth of 230Th and 231Pa due to the decay of the natural 234U and 235U preserved in the acidified samples during the period of time between sample collection and U-Th/Pa separation during anion exchange chromatography. Thus, the reported 230Th and 231Pa concentrations have been corrected to represent their concentrations at the time of sampling. U concentrations in the samples were estimated using the bottle salinity (S) measured from the CTD and the U-Salinity relationship in seawater (Owens et al. 2011), [U] = (0.100 * S – 0.326) ng U (g seawater)-1. We used seawater U-isotopic compositions of 234U/238 U = 1.1468 activity ratio (Andersen et al., 2010), and 238U/235U = 137.824 mole ratio (Weyer et al., 2008), to calculate [234U] and [235U] respectively based on [U].
Individual uncertainties for thorium were calculated to include contributions from (a) blank correction using the variance of the blanks measured over the course of the analyses, (b) standard error of the ratios of the analysis (typically close to counting statistics) and (c) spike calibration. For Pa we also included assessment of the correction from the yield correction, mass bias and machine background. In order to assess the reproducibility of the procedure, repeat analyses were performed on the GEOTRACES 2010-1 standard. For standards run with the GEOTRACES intercalibration and section samples the reproducibility for each isotope was 0.9% for 230Th, 0.8% for 232Th and 3.6% for 231Pa.
Primary references for this data set:
Hayes, C.T., Anderson, R.F., Fleisher, M.Q., Huang, K.-F., Robinson, L.F., Lu, Y., Cheng, H., Edwards, R.L. and Moran, S.B, 2015. 230Th and 231Pa on GEOTRACES GA03, the U.S. GEOTRACES North Atlantic transect, and implications for modern and paleoceanographic chemical fluxes. Deep-Sea Res. II 116, 29-41.
Hayes, C.T., Anderson, R.F., Fleisher, M.Q., Vivancos, S.M., Lam, P.J., Ohnemus, D.C., Huang, K.-F., Robinson, L.F., Lu, Y., Cheng, H., Edwards, R.L. and Moran, S.B., 2015. Intensity of Th and Pa scavenging partitioned by particle chemistry in the North Atlantic Ocean. Marine Chemistry 170, 49-60.
Additional references:
Andersen, M.B., Stirling, C.H., Zimmermann, B., Halliday, A.N., 2010. Precise determination of the open ocean 234U/238U composition. Geochem. Geophys. Geosyst. 11, Q12003.
Anderson, R.F., Fleisher, M.Q., Robinson, L.F., Edwards, R.L., Hoff, J., Moran, S.B., Rutgers van der Loeff, M.M., Thomas, A.L., Roy-Barman, M., François, R., 2012. GEOTRACES intercalibration of 230Th, 232Th, 231Pa, and prospects for 10Be. Limnol. Oceanogr. Methods 10, 179-213.
Auro ME, LF Robinson, A Burke, LI Bradtmiller, MQ Fleisher, RF Anderson Improvements to 232-thorium, 230-thorium, and 231-protactinium analysis in seawater arising from GEOTRACES intercalibration Limnology and Oceanography, Methods 10, 464-474.
Chen, J.H., Edwards, R.L., Wasserburg, G.J., 1986. 238U, 234U and 232Th in seawater. Earth Planet. Sci. Lett. 80, 241-251.
Chen, J.H., Lawrence Edwards, R., Wasserburg, G.J., 1986. 238U, 234U and 232Th in seawater. Earth Planet. Sci. Lett. 80, 241-251.
Cheng, H., Edwards, R.L., Hoff, J., Gallup, C.D., Richards, D.A., and Asmerom, Y. 2000. The half-lives of uranium-234 and thorium-230. Chemical Geology 169, 17-33.
Cheng, H., Edwards, R.L., Shen, C.-C., Polyak, V.J., Asmerom, Y., Woodhead, J., Hellstrom, J., Wang, Y.J., Kong, X.G., SpÖtl, C., Wang, X.F., and E.Calvin Alexander Jr. 2013. Improvements in 230Th dating, 230Th and 234U half-life values, and U–Th isotopic measurements by multi-collector inductively coupled plasma mass spectrometry. Earth and Planetary Science Letters 371-372, 82-91.
Lam, P.J., Ohnemus, D.C. and Auro, M.E., 2015. Size-fractionated major particle composition and concentrations from the US GEOTRACES North Atlantic Zonal Transect. Deep-Sea Res. II 116, 303-320.
Owens, S.A., Buesseler, K.O., Sims, K.W.W., 2011. Re-evaluating the 238U-salinity relationship in seawater: Implications for the 238U–234Th disequilibrium method. Marine Chemistry 127, 31-39.
Rev. Geophys. 33 (2), 241–265.
Roy-Barman, M., Coppola, L. and Souhaut, M. (2002) Thorium isotopes in the western Mediterranean Sea: an insight into the marine particle dynamics. Earth Planet. Sci. Lett. 196, 161-174.
Shen, C.-C., Cheng, H., Edwards, R.L., Thomas, R.B. and Moran, S.B. 2003. Attogram-sized 231Pa Analysis in Dissolved and Particulate fractions of Seawater by Thermal Ionization Mass Spectroscopy. Analytical Chemistry v. 75, issue 5, 1075-1079.
Shen, C.-C., Edwards, R.L., Cheng, H., Dorale, J.A., Thomas, R.B., Moran, S.B., Weinstein, S., and Edmonds, H.N. 2002. Uranium and thorium isotopic and concentration measurements by magnetic sector inductively coupled plasma mass spectrometry. Chemical Geology 185. no. 3-4, 165-178.
Shen, C.C., Wu, C.C., Cheng, H., Edwards, R.L., Hsieh, Y.T., Gallet, S., Chang, C.C., Li, T.Y., Lam, D.D., Kano, A, Hori, M. and Spotl, C. 2012. High-precision and high-resolution carbonate Th-230 dating by MC-ICP-MS with SEM protocols. Geochimica Cosmochim. Acta 99, 71-86. DOI: 10.1016/j.gca.2012.09.018.
Taylor, S.R., McLennan, S.M., 1995. The geochemical evolution of the continental crust.
Weyer, S., Anbar, A.D., Gerdes, A., Gordon, G.W., Algeo, T.J., Boyle, E.A., 2008. Natural fractionation of 238U/235U. Geochim. Cosmochim. Acta 72, 345-359.
Additional GEOTRACES Processing performed by BCO-DMO:
After the data were submitted to the International Data Management Office, BODC, the office noticed that important identifying information was missing in many datasets. With the agreement of BODC and the US GEOTRACES lead PIs, BCO-DMO added standard US GEOTRACES information, such as the US GEOTRACES event number, to each submitted dataset lacking this information. To accomplish this, BCO-DMO compiled a 'master' dataset composed of the following parameters: station_GEOTRC, cast_GEOTRC (bottle and pump data only), event_GEOTRC, sample_GEOTRC, sample_bottle_GEOTRC (bottle data only), bottle_GEOTRC (bottle data only), depth_GEOTRC_CTD (bottle data only), depth_GEOTRC_CTD_rounded (bottle data only), BTL_ISO_DateTime_UTC (bottle data only), and GeoFish_id (GeoFish data only). This added information will facilitate subsequent analysis and inter comparison of the datasets.
Bottle parameters in the master file were taken from the GT-C_Bottle_GT10, GT-C_Bottle_GT11, ODF_Bottle_GT10, and ODF_Bottle_GT11 datasets. Non-bottle parameters, including those from GeoFish tows, Aerosol sampling, and McLane Pumps, were taken from the Event_Log_GT10 and Event_Log_GT11 datasets. McLane pump cast numbers missing in event logs were taken from the Particulate Th-234 dataset submitted by Ken Buesseler.
A standardized BCO-DMO method (called “join”) was then used to merge the missing parameters to each US GEOTRACES dataset, most often by matching on sample_GEOTRC or on some unique combination of other parameters.
If the master parameters were included in the original data file and the values did not differ from the master file, the original data columns were retained and the name of the parameters were changed from the PI-submitted names to the standardized master names. If there were differences between the PI-supplied parameter values and those in the master file, both columns were retained. If the original data submission included all of the master parameters, no additional columns were added, but parameter names were modified to match the naming conventions of the master file.
See the dataset parameters documentation for a description of which parameters were supplied by the PI and which were added via the join method.
- Version 4b: 16 May 2016; metadata text revised.
- Version 4: 29 Sep 2015; combined both dissolved and particulate Th & Pa datasets into one.
- Version 3: 17 Mar 2014; revised dataset Th&Pa dissolved
- Version 2: 7 Nov. 2013
- Version 1: 9 Jan 2013
| File |
|---|
ThPa_all_GT10-11_v4.csv (Comma Separated Values (.csv), 220.93 KB) MD5:d357a60518b2d4bb6b9767e0e332c29a Primary data file for dataset ID 3847 |
| Parameter | Description | Units |
| cruise_id | Official cruise identifier e.g. KN199-04 = R/V Knorr cruise number 199-04. | text |
| cruise_part | Identifier for a segment of a leg of a cruise where a leg may have been broken into parts: For KN204-01: part A = 11/6/2011 to 11/18/2011 (Woods Hole to Bermuda) +part B = 11/19/2011 to 12/11/2011 (Bermuda to Praia-- Cabo Verde) | A or B |
| station_GEOTRC | GEOTRACES station number; ranges from 1 through 12 for KN199-04 and 1 through 24 for KN204-01. Stations 7 and 9 were skipped on KN204-01. PI-supplied values were identical to those in the intermediate US GEOTRACES master file. Originally submitted as 'station'; this parameter name has been changed to conform to BCO-DMO's GEOTRACES naming conventions. | unitless |
| lat_sta | Nominal station latitude for mapping; north is positive | decimal degrees |
| lon_sta | Nominal station longitude for mapping; east is positive | decimal degrees |
| depth_GEOTRC | Observation/sample depth in meters; Niskin sample depth calculated from CTD pressure. PI-supplied values were identical to those in the intermediate US GEOTRACES master file. Originally submitted as 'depth'; this parameter name has been changed to conform to BCO-DMO's GEOTRACES naming conventions. | meters |
| cast_GEOTRC | Cast identifier numbered consecutively within a station. PI-supplied values were identical to those in the intermediate US GEOTRACES master file. Originally submitted as 'cast'; this parameter name has been changed to conform to BCO-DMO's GEOTRACES naming conventions. | integer |
| event_GEOTRC | Unique identifying number for US GEOTRACES sampling events; ranges from 2001 to 2225 for KN199-04 events and from 3001 to 3282 for KN204-01 events. PI-supplied values were identical to those in the intermediate US GEOTRACES master file. Originally submitted as 'event'; this parameter name has been changed to conform to BCO-DMO's GEOTRACES naming conventions. | integer |
| date_collected | Date sample collected; UTC | yyyymmdd |
| time | Time sample collected; UTC | hhmm |
| bottle_GEOTRC | Alphanumeric characters identifying bottle type (e.g. NIS representing Niskin and GF representing GOFLO) and position on a CTD rosette. PI-supplied values were identical to those in the intermediate US GEOTRACES master file. Originally submitted as 'bottle'; this parameter name has been changed to conform to BCO-DMO's GEOTRACES naming conventions. | text-integer |
| sample_GEOTRC | Unique identifying number for US GEOTRACES samples; ranges from 5033 to 6078 for KN199-04 and from 6112 to 8148 for KN204-01. PI-supplied values were identical to those in the intermediate US GEOTRACES master file Originally submitted as 'sample'; this parameter name has been changed to conform to BCO-DMO's GEOTRACES naming conventions. | integer |
| lab | lab group that analyzed the samples: LDEO=Lamont Doherty Earth Observatory; WHOI=Woods Hole Oceanographic Inst.; UMN=Univ. of Minnesota | text |
| date_dissolved_U_separation | Date when dissolved U separated from Th and Pa (used for correcting measured dissolved Th-230 and Pa-231 for ingrowth by decay of dissolved uranium during the time between sample collection and U separation | yyyymmdd |
| Th_232_D_CONC_BOTTLE | Dissolved Th-232 concentration | pmol/kg |
| Th_232_D_CONC_BOTTLE_ERR | 1 sigma error in Dissolved Th-232 | pmol/kg |
| Th_232_D_CONC_BOTTLE_FLAG | Flag for Dissolved Th-232: 1 = good; 2 = questionable; 3 = bad or no data | unitless |
| Th_230_D_CONC_BOTTLE | Dissolved Th-230 concentration | uBq/kg |
| Th_230_D_CONC_BOTTLE_ERR | 1 sigma error in Dissolved Th-230 | uBq/kg |
| Th_230_D_CONC_BOTTLE_FLAG | Flag for Dissolved Th-230: 1 = good; 2 = questionable; 3 = bad or no data | unitless |
| Pa_231_D_CONC_BOTTLE | Dissolved Pa-231 concentration | uBq/kg |
| Pa_231_D_CONC_BOTTLE_ERR | 1 sigma error in Dissolved Pa-231 | uBq/kg |
| Pa_231_D_CONC_BOTTLE_FLAG | Flag for Dissolved Pa-231: 1 = good; 2 = questionable; 3 = bad or no data | unitless |
| Th_232_SP_CONC_PUMP | Small particulate Th-232 concentration | pmol/kg |
| Th_232_SP_CONC_PUMP_ERR | 1 sigma error in small particulate Th-232 | pmol/kg |
| Th_232_SP_CONC_PUMP_FLAG | Flag for small particulate Th-232: 1 = good; 2 = questionable; 3 = bad or no data | unitless |
| Th_230_SP_CONC_PUMP | Small particulate Th-230 concentration | uBq/kg |
| Th_230_SP_CONC_PUMP_ERR | 1 sigma error in small particulate Th-230 | uBq/kg |
| Th_230_SP_CONC_PUMP_FLAG | Flag for small particulate Th-230: 1 = good; 2 = questionable; 3 = bad or no data | unitless |
| Pa_231_SP_CONC_PUMP | Small particulate Pa-231 concentration | uBq/kg |
| Pa_231_SP_CONC_PUMP_ERR | 1 sigma error in small particulate Pa-231 | uBq/kg |
| Pa_231_SP_CONC_PUMP_FLAG | Flag for small particulate Pa-231: 1 = good; 2 = questionable; 3 = bad or no data | unitless |
| Th_232_LP_CONC_PUMP | Large particulate Th-232 concentration | pmol/kg |
| Th_232_LP_CONC_PUMP_ERR | 1 sigma error in large particulate Th-232 | pmol/kg |
| Th_232_LP_CONC_PUMP_FLAG | Flag for large particulate Th-232: 1 = good; 2 = questionable; 3 bad or no data | unitless |
| Th_230_LP_CONC_PUMP | Large particulate Th-230 concentration | uBq/kg |
| Th_230_LP_CONC_PUMP_ERR | 1 sigma error in large particulate Th-230 | uBq/kg |
| Th_230_LP_CONC_PUMP_FLAG | Flag for large particulate Th-230: 1 = good; 2 = questionable; 3 bad or no data | unitless |
| Pa_231_LP_CONC_PUMP | Large particulate Pa-231 concentration | uBq/kg |
| Pa_231_LP_CONC_PUMP_ERR | 1 sigma error in large particulate Pa-231 | uBq/kg |
| Pa_231_LP_CONC_PUMP_FLAG | Flag for large particulate Pa-231: 1 = good; 2 = questionable; 3 bad or no data | unitless |
| Th_230_D_XS_CONC_BOTTLE | Dissolved Th-230 concentration corrected for the dissolution of lithogenic minerals; thereby isolating the dissolved Th-230 produced by decay of dissolved uranium | uBq/kg |
| Th_230_D_XS_CONC_BOTTLE_ERR | Dissolved Th-230 concentration error | uBq/kg |
| Pa_231_D_XS_CONC_BOTTLE | Dissolved Pa-231 concentration corrected for the dissolution of lithogenic minerals (see notes for full explanation) | uBq/kg |
| Pa_231_D_XS_CONC_BOTTLE_RR | Dissolved Pa-231 concentration error | uBq/kg |
| Th_230_SP_ADS_CONC_PUMP | Small particulate adsorbed Th-230 (see notes for full explanation) | uBq/kg |
| Th_230_SP_ADS_CONC_PUMP_ERR | Small particulate adsorbed Th-230 error | uBq/kg |
| Th_230_SP_XS_CONC_PUMP | Small particulate excess Th-230 (see notes for full explanation) | uBq/kg |
| Th_230_SP_XS_CONC_PUMP_ERR | Small particulate excess Th-230 error | uBq/kg |
| Pa_231_SP_ADS_CONC_PUMP | Small particulate adsorbed Pa-231 (see notes for full explanation) | uBq/kg |
| Pa_231_SP_ADS_CONC_PUMP_ERR | Small particulate adsorbed Pa-231 error | uBq/kg |
| Pa_231_SP_XS_CONC_PUMP | Small particulate excess Pa-231 (see notes for full explanation) | uBq/kg |
| Pa_231_SP_XS_CONC_PUMP_ERR | Small particulate excess Pa-231 error | uBq/kg |
| Th_230_LP_ADS_CONC_PUMP | Large particulate adsorbed Th-230 (see notes for full explanation) | uBq/kg |
| Th_230_LP_ADS_CONC_PUMP_ERR | Large particulate adsorbed Th-230 error | uBq/kg |
| Th_230_LP_XS_CONC_PUMP | Large particulate excess Th-230 (see notes for full explanation) | uBq/kg |
| Th_230_LP_XS_CONC_PUMP_ERR | Large particulate excess Th-230 error | uBq/kg |
| Pa_231_LP_ADS_CONC_PUMP | Large particulate adsorbed Pa-231 (see notes for full explanation) | uBq/kg |
| Pa_231_LP_ADS_CONC_PUMP_ERR | Large particulate adsorbed Pa-231 error | uBq/kg |
| Pa_231_LP_XS_CONC_PUMP | Large particulate excess Pa-231 (see notes for full explanation) | uBq/kg |
| Pa_231_LP_XS_CONC_PUMP_ERR | Large particulate excess Pa-231 error | uBq/kg |
| Dataset-specific Instrument Name | CTD Sea-Bird |
| Generic Instrument Name | CTD Sea-Bird |
| Dataset-specific Description | a Sea-Bird Electronics CTD carousel fitted with 12 30-liter PVC Niskin bottles, maintained and operated by the Ocean Data Facility of Scripps Institution of Oceangraphy. The carousel was lowered from the ship with steel wire. Niskin bottles were equipped with nylon-coated closure springs and Viton O-rings. |
| Generic Instrument Description | A Conductivity, Temperature, Depth (CTD) sensor package from SeaBird Electronics. This instrument designation is used when specific make and model are not known or when a more specific term is not available in the BCO-DMO vocabulary. Refer to the dataset-specific metadata for more information about the specific CTD used. More information from: http://www.seabird.com/ |
| Dataset-specific Instrument Name | GeoFish |
| Generic Instrument Name | GeoFish Towed near-Surface Sampler |
| Generic Instrument Description | The GeoFish towed sampler is a custom designed near surface ( |
| Dataset-specific Instrument Name | Mass Spectrometer |
| Generic Instrument Name | Mass Spectrometer |
| Dataset-specific Description | For the Dissolved Th & Pa data: Thermo-Finnigan Neptune mass spectrometer
For the Particulate Th & Pa data: Thermo Scientific Element XR Inductively-couple plasma mass spectrometer (ICP-MS) |
| Generic Instrument Description | General term for instruments used to measure the mass-to-charge ratio of ions; generally used to find the composition of a sample by generating a mass spectrum representing the masses of sample components. |
| Dataset-specific Instrument Name | |
| Generic Instrument Name | McLane Pump |
| Dataset-specific Description | McLane Research in-situ pumps (WTS-LV) that had been modified to accommodate two flowpaths (Lam and Morris Patent pending). The wire-out was used to target depths during deployment, and a self-recording Seabird 19plus CTD deployed at the end of the line and RBR data loggers attached to three of the eight pumps were used to correct for actual depths during pumping.
Samples were collected with the pump over paired 0.8um Pall Supor800 polyethersulfone filters behind a 51 um Sefar polyester mesh prefilter (See sampling Methodology for details).
142 mm-diameter "mini-MULVFS" style filter holders with two stages for two size fractions and multiple baffle systems designed to ensure even particle distribution and prevent particle loss (Bishop et al. 2012). One of two filter holder/flowpaths was loaded with a 51um Sefar polyester mesh prefilter followed by paired 0.8 um Pall Supor800 polyethersulfone filters. |
| Generic Instrument Description | McLane pumps sample large volumes of seawater at depth. They are attached to a wire and lowered to different depths in the ocean. As the water is pumped through the filter, particles suspended in the ocean are collected on the filters. The pumps are then retrieved and the contents of the filters are analyzed in a lab. |
| Dataset-specific Instrument Name | Niskin bottle |
| Generic Instrument Name | Niskin bottle |
| Dataset-specific Description | Twelve 30-liter PVC Niskin bottles, maintained and operated by the Ocean Data Facility of Scripps Institution of Oceangraphy. |
| Generic Instrument Description | A Niskin bottle (a next generation water sampler based on the Nansen bottle) is a cylindrical, non-metallic water collection device with stoppers at both ends. The bottles can be attached individually on a hydrowire or deployed in 12, 24, or 36 bottle Rosette systems mounted on a frame and combined with a CTD. Niskin bottles are used to collect discrete water samples for a range of measurements including pigments, nutrients, plankton, etc. |
KN199-04
| Website | |
| Platform | R/V Knorr |
| Report | |
| Start Date | 2010-10-15 |
| End Date | 2010-11-04 |
| Description | This cruise constitutes the first survey section as part of the U.S. participation in an international program named GEOTRACES.
Funding: NSF OCE award 0926423
Science Objectives: To obtain state of the art trace metal and isotope measurements on a suite of samples taken on a mid-latitude zonal transect of the North Atlantic. In particular, sampling targeted the oxygen minimum zone extending off the west African coast near Mauritania, the TAG hydrothermal field, and the western boundary current system along Line W. For additional information, please refer to the GEOTRACES program Web site (https://www.geotraces.org/) for overall program objectives and a summary of properties measured.
Science Activities include seawater sampling via GoFLO and Niskin carousels, in situ pumping (and filtration), CTDO2 and transmissometer sensors, underway pumped sampling of surface waters, and collection of aerosols and rain. Hydrography, CTD and nutrient measurements were supported by the Ocean Data Facility (J. Swift) at Scripps Institution of Oceanography and funded through NSF Facilities. They provided an additional CTD rosette system along with nephelometer and LADCP. A trace metal clean Go-Flo Rosette and winch were provided by the group at Old Dominion University (G. Cutter) along with a towed underway pumping system.
Additional cruise information is available from the Rolling Deck to Repository (R2R): https://www.rvdata.us/search/cruise/KN199-04
Other Relevant Links:
List of cruise participants: [ PDF ]
Cruise track: JPEG image (from Woods Hole Oceanographic Institution, vessel operator)
ADCP data are available from the Currents ADCP group at the University of Hawaii: KN199-04 ADCP |
KN204-01
| Website | |
| Platform | R/V Knorr |
| Report | |
| Start Date | 2011-11-06 |
| End Date | 2011-12-11 |
| Description | The US GEOTRACES North Atlantic cruise aboard the R/V Knorr completed the section between Lisbon and Woods Hole that began in October 2010 but was rescheduled for November-December 2011. The R/V Knorr made a brief stop in Bermuda to exchange samples and personnel before continuing across the basin. Scientists disembarked in Praia, Cape Verde, on 11 December. The cruise was identified as KN204-01A (first part before Bermuda) and KN204-01B (after the Bermuda stop). However, the official deployment name for this cruise is KN204-01 and includes both part A and B.
Science activities included: ODF 30 liter rosette CTD casts, ODU Trace metal rosette CTD casts, McLane particulate pump casts, underway sampling with towed fish and sampling from the shipboard "uncontaminated" flow-through system.
Full depth stations are shown in the accompanying figure (see below). Additional stations to sample for selected trace metals to a depth of 1000 m are not shown. Standard stations are shown in red (as are the ports) and "super" stations, with extra casts to provide large-volume samples for selected parameters, are shown in green.
Station spacing is concentrated along the western margin to evaluate the transport of trace elements and isotopes by western boundary currents. Stations across the gyre will allow scientists to examine trace element supply by Saharan dust, while also contrasting trace element and isotope distributions in the oligotrophic gyre with conditions near biologically productive ocean margins, both in the west, to be sampled now, and within the eastern boundary upwelling system off Mauritania, sampled last year.
Funding: The cruise was funded by NSF OCE awards 0926204, 0926433 and 0926659.
Additional cruise information is available from the Rolling Deck to Repository (R2R): https://www.rvdata.us/search/cruise/KN204-01
Other Relevant Links:
ADCP data are available from the Currents ADCP group at the University of Hawaii at the links below:
KN204-01A (part 1 of 2011 cruise; Woods Hole, MA to Bermuda)
KN204-01B (part 2 of 2011 cruise; Bermuda to Cape Verde) |
U.S. GEOTRACES North Atlantic Transect (GA03) (U.S. GEOTRACES NAT)
Much of this text appeared in an article published in OCB News, October 2008, by the OCB Project Office.
The first U.S. GEOTRACES Atlantic Section will be specifically centered around a sampling cruise to be carried out in the North Atlantic in 2010. Ed Boyle (MIT) and Bill Jenkins (WHOI) organized a three-day planning workshop that was held September 22-24, 2008 at the Woods Hole Oceanographic Institution. The main goal of the workshop, sponsored by the National Science Foundation and the U.S. GEOTRACES Scientific Steering Committee, was to design the implementation plan for the first U.S. GEOTRACES Atlantic Section. The primary cruise design motivation was to improve knowledge of the sources, sinks and internal cycling of Trace Elements and their Isotopes (TEIs) by studying their distributions along a section in the North Atlantic (Figure 1). The North Atlantic has the full suite of processes that affect TEIs, including strong meridional advection, boundary scavenging and source effects, aeolian deposition, and the salty Mediterranean Outflow. The North Atlantic is particularly important as it lies at the "origin" of the global Meridional Overturning Circulation.
It is well understood that many trace metals play important roles in biogeochemical processes and the carbon cycle, yet very little is known about their large-scale distributions and the regional scale processes that affect them. Recent advances in sampling and analytical techniques, along with advances in our understanding of their roles in enzymatic and catalytic processes in the open ocean provide a natural opportunity to make substantial advances in our understanding of these important elements. Moreover, we are motivated by the prospect of global change and the need to understand the present and future workings of the ocean's biogeochemistry. The GEOTRACES strategy is to measure a broad suite of TEIs to constrain the critical biogeochemical processes that influence their distributions. In addition to these "exotic" substances, more traditional properties, including macronutrients (at micromolar and nanomolar levels), CTD, bio-optical parameters, and carbon system characteristics will be measured. The cruise starts at Line W, a repeat hydrographic section southeast of Cape Cod, extends to Bermuda and subsequently through the North Atlantic oligotrophic subtropical gyre, then transects into the African coast in the northern limb of the coastal upwelling region. From there, the cruise goes northward into the Mediterranean outflow. The station locations shown on the map are for the "fulldepth TEI" stations, and constitute approximately half of the stations to be ultimately occupied.
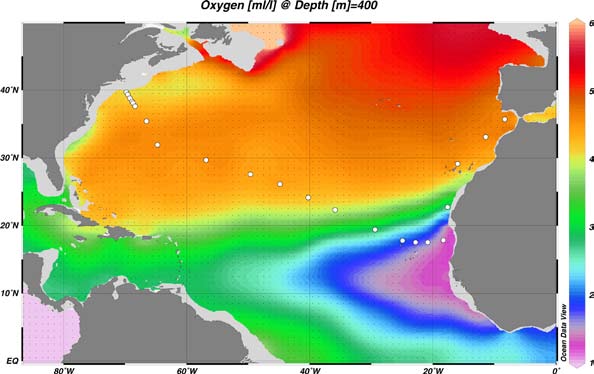
Figure 1. The proposed 2010 Atlantic GEOTRACES cruise track plotted on dissolved oxygen at 400 m depth. Data from the World Ocean Atlas (Levitus et al., 2005) were plotted using Ocean Data View (courtesy Reiner Schlitzer). [click on the image to view a larger version]
Hydrography, CTD and nutrient measurements will be supported by the Ocean Data Facility (J. Swift) at Scripps Institution of Oceanography and funded through NSF Facilities. They will be providing an additional CTD rosette system along with nephelometer and LADCP. A trace metal clean Go-Flo Rosette and winch will be provided by the group at Old Dominion University (G. Cutter) along with a towed underway pumping system.
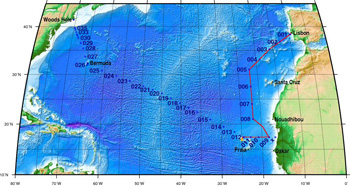
The North Atlantic Transect cruise began in 2010 with KN199 leg 4 (station sampling) and leg 5 (underway sampling only) (Figure 2).
Figure 2. The red line shows the cruise track for the first leg of the US Geotraces North Atlantic Transect on the R/V Knorr in October 2010. The rest of the stations (beginning with 13) will be completed in October-December 2011 on the R/V Knorr (courtesy of Bill Jenkins, Chief Scientist, GNAT first leg). [click on the image to view a larger version]
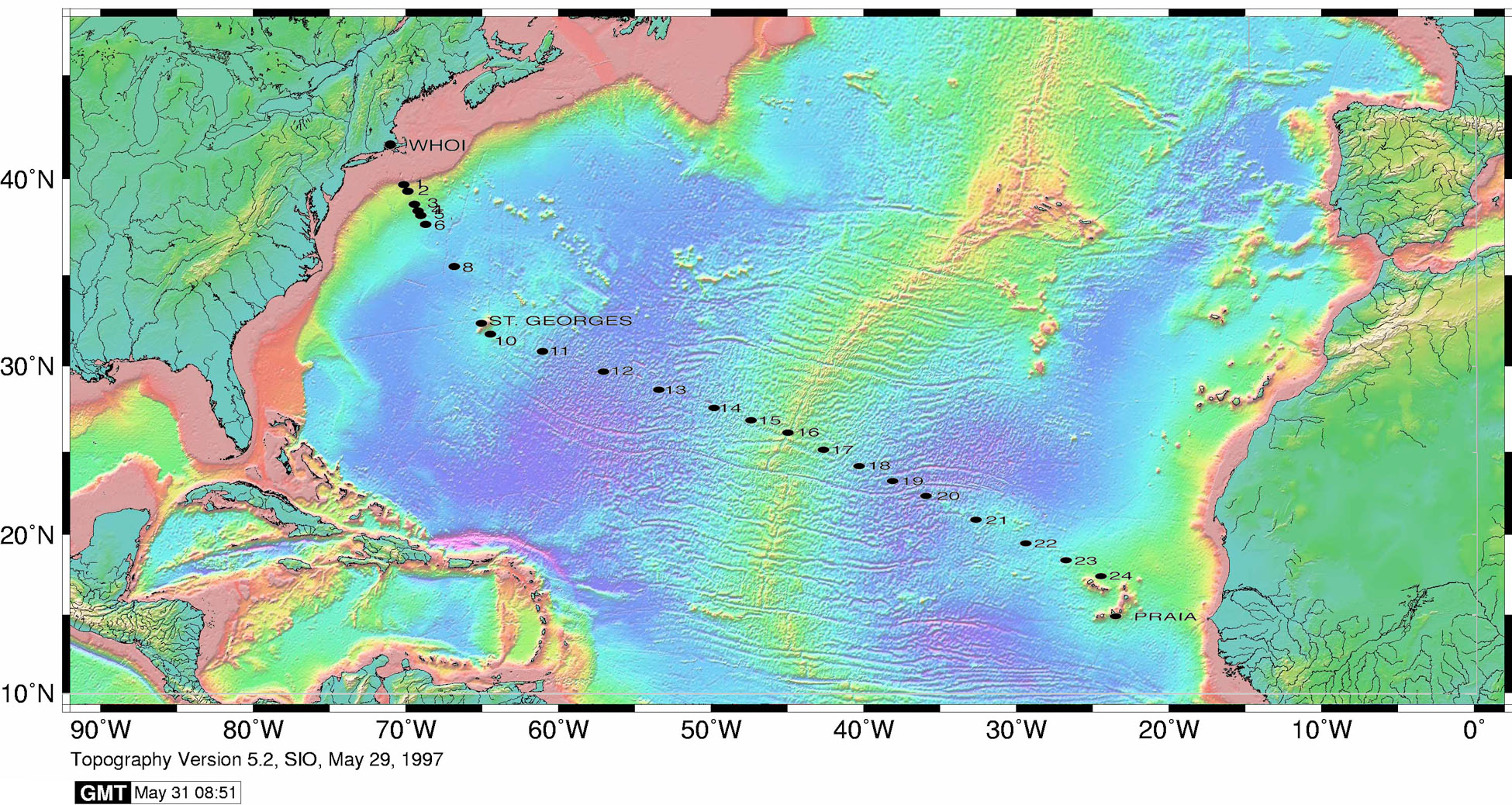
The section completion effort resumed again in November 2011 with KN204-01A,B (Figure 3).
KN204-01A,B Cruise Report (PDF)
Figure 3. Station locations occupied on the US Geotraces North Atlantic Transect on the R/V Knorr in November 2011. [click on the image to view a larger version]
Data from the North Atlantic Transect cruises are available under the Datasets heading below, and consensus values for the SAFe and North Atlantic GEOTRACES Reference Seawater Samples are available from the GEOTRACES Program Office: Standards and Reference Materials
ADCP data are available from the Currents ADCP group at the University of Hawaii at the links below:
KN199-04 (leg 1 of 2010 cruise; Lisbon to Cape Verde)
KN199-05 (leg 2 of 2010 cruise; Cape Verde to Charleston, NC)
KN204-01A (part 1 of 2011 cruise; Woods Hole, MA to Bermuda)
KN204-01B (part 2 of 2011 cruise; Bermuda to Cape Verde)
U.S. GEOTRACES (U.S. GEOTRACES)
GEOTRACES is a SCOR sponsored program; and funding for program infrastructure development is provided by the U.S. National Science Foundation.
GEOTRACES gained momentum following a special symposium, S02: Biogeochemical cycling of trace elements and isotopes in the ocean and applications to constrain contemporary marine processes (GEOSECS II), at a 2003 Goldschmidt meeting convened in Japan. The GEOSECS II acronym referred to the Geochemical Ocean Section Studies To determine full water column distributions of selected trace elements and isotopes, including their concentration, chemical speciation, and physical form, along a sufficient number of sections in each ocean basin to establish the principal relationships between these distributions and with more traditional hydrographic parameters;
* To evaluate the sources, sinks, and internal cycling of these species and thereby characterize more completely the physical, chemical and biological processes regulating their distributions, and the sensitivity of these processes to global change; and
* To understand the processes that control the concentrations of geochemical species used for proxies of the past environment, both in the water column and in the substrates that reflect the water column.
GEOTRACES will be global in scope, consisting of ocean sections complemented by regional process studies. Sections and process studies will combine fieldwork, laboratory experiments and modelling. Beyond realizing the scientific objectives identified above, a natural outcome of this work will be to build a community of marine scientists who understand the processes regulating trace element cycles sufficiently well to exploit this knowledge reliably in future interdisciplinary studies.
Expand "Projects" below for information about and data resulting from individual US GEOTRACES research projects.
| Funding Source | Award |
|---|---|
| NSF Division of Ocean Sciences (NSF OCE) | |
| NSF Division of Ocean Sciences (NSF OCE) | |
| NSF Division of Ocean Sciences (NSF OCE) |
[ table of contents | back to top ]