Sulfate reduction energetics at Main Endeavor grotto chimney from samples collected on RV Atlantis (AT18-08) during Jason II dives in the Juan de Fuca Ridge from July to August 2011
Project
Program
| Contributors | Affiliation | Role |
|---|---|---|
| Girguis, Peter | Harvard University | Principal Investigator |
| Rogers, Karyn | Rensselaer Polytechnic Institute (RPI) | Co-Principal Investigator |
| Frank, Kiana | University of Hawaiʻi at Mānoa (SOEST) | Contact |
| Ake, Hannah | Woods Hole Oceanographic Institution (WHOI BCO-DMO) | BCO-DMO Data Manager |
Abstract
The effects of key environmental variables (temperature, pH, H2S, SO42-, DOC) on sulfate reduction energetics in material recovered from a hydrothermal flange from the Grotto edifice in the Main Endeavor Field, Juan de Fuca Ridge. Sulfate reduction was measured in batch reactions across a range of physico-chemical conditions. Temperature and pH were the strongest stimuli, and maximum sulfate reduction rates were observed at 50 degrees celsius and pH 6.
Information for this dataset was derived from single massive piece of hydrothermal deposit (approximately ~100 kg in weight) that was recovered from a flange on the Grotto vent (47.949, -129.098) at a depth of 2188.3 m (Dive J2-575, AT-18-08, R/V Atlantis) and brought up to the surface in the basket of the ROV Jason II.
Methodology for this dataset is from: Frank et al., 2015
Tables and Figures referenced in the acquisition description are found in the paper Frank et al., 2015
For each independent treatment, aliquots of 7.5 mL flange slurry (approx. 29 g wet weight and 20 g dry weight) were transferred into Balch tubes in an anaerobic chamber, and supplemented with 15 mL of sterile artificial seawater media designed to mimic the geochemical conditions within a hydrothermal flange (400 mM NaCl, 25 mM KCl, 30 mM CaCl2, 2.3 mM NaHCO3, 14 mM NaSO42-, 1 mM H2S, and 50 uM dissolved organic carbon - consisting of equimolar proportions 10 uM of pyruvate, citrate, formate, acetate, lactate) under a pure nitrogen headspace.
Concentrations of sulfide, sulfate and dissolved organic carbon (DOC) were varied independently to investigate concentration dependent effects on the rates of SR. The range of experimental conditions tested was determined from previously published concentration profiles of aqueous species modeled as functions of temperature and position within the Grotto vent structure (Tivey, 2004). Concentrations were varied by orders of magnitude within the modeled ranges to simulate conditions representative of different mixing regimes between seawater and vent fluid (Table 1). The range of DOC (which we approximate as a mix of pyruvate, citrate, formate, acetate, lactate – most of which have been identified to varying degrees within vent fluid and are known carbon sources for heterotrophic SR in culture) concentrations tested were based on the average DOC concentrations measured within diffuse fluids at the Main Endeavor Field (Lang et al., 2006; Lang et al., 2010). Hydrogen sulfide was present as H2S (pKa in seawater of 6.60) across all the conditions tested (Amend & Shock, 2001). Incubations were carried out at pH 4 (to simulate the pH of end-member Grotto vent fluid and the average calculated pH of mixed fluids in highly reduced zones within the flange; Tivey 2004) as well as pH 6 (representative of the calculated pH in fluid mixing zones; Tivey 2004). All the results are presented and discussed in the context of the initial measured media conditions.
Tables and Figures referenced in the processing description are found in the paper Frank et al., 2015
Potential energy yields of the different metabolisms available in the incubations depend on temperature and fluid compositions. To quantify the energy yield from heterotrophic sulfate reduction (Table 2) in each incubation values of overall Gibbs energy () were calculated according to:
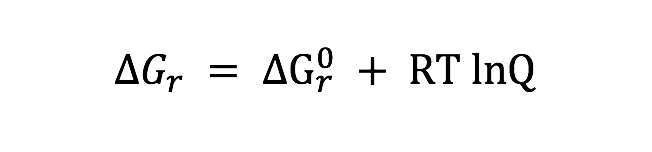
where is the standard Gibbs energy of reaction at in situ temperature and pressure conditions, R is the gas constant, T is the temperature (Kelvin), and Q is the activity product, defined as
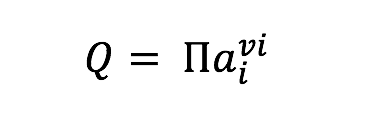
where ai represents the activity of the ith species and vi is the stoichiometric reaction coefficient, which is positive for products and negative for reactants. Values of were calculated at 1 bar and incubation temperatures using the geochemical software package SUPCRT92 (Johnson et al., 1992) and additional thermodynamic data from (Shock, 1995). Activities of aqueous species were calculated using the geochemical speciation program EQ3 (Wolery, 1992) based on the media composition described in section 2.2 and Table 1, with additional data from previously published work (Shock, 1995; Shock & Koretsky, 1993). For concentrations equal to zero, a value of 10-13 mol/kg was used as input. Resulting aqueous activities were used to calculate values of normalized for the number of electrons transferred in the redox for the reactions in Table 2. These reflect the metabolic energy available at the start of each incubation experiment for the complete oxidation of each organic acid, metabolisms that are documented among known sulfate reducers (Amend and Shock, 2001). Furthermore, to calculate the energy density in each incubation (as in Amend et al., 2011), it was assumed that the amended organic acids were the limiting reactant for all experiments when sulfate concentrations were in excess of 1 mM; otherwise sulfate was assumed to be limiting. While some sulfate reducers are known to produce carboxylic acid and alcohol intermediates, incomplete oxidation reactions were not considered here, as the goal of these calculations was to generate a broad understanding of sulfate reduction energetics, and not the metabolic potential for a particular species. Such an approach is common when comparing microbial metabolisms independent of species-specific pathways (e.g. Amend et al., 2004; Rogers & Amend, 2006; Skoog et al., 2007), although it should be noted that incomplete oxidation (fermentation) generally yields much less energy than complete oxidation (Rogers & Amend, 2006; Skoog et al., 2007).
To account for potential interactions between chimney-derived trace metals and amended sulfide, the saturation states of sulfide minerals were calculated as part of the initial fluid speciation. Using reported concentrations of relevant trace metals (Fe, Zn, Cu, etc.) in end-member Grotto hydrothermal fluid (Butterfield et al., 1994), maximum aqueous activities of trace metals were calculated with the EQ3 geochemical speciation program (EQ3/6 1998; EQ3NR 1998). Several sulfide minerals commonly found in hydrothermal chimneys (e.g. pyrite, chalcocite, sphalerite) were supersaturated under incubation conditions, particularly for incubations with high concentrations of amended sulfide. The irreversible abiotic precipitation of mineral sulfides has the potential to draw down aqueous sulfide concentrations and impact sulfate reductions rates. Therefore, the geochemical reaction path program EQ6 (EQ3/6 1998; EQ6 1998) was used to constrain fluid compositions to equilibrium with these minerals phases. Using the single point model in EQ6, the Gibbs energy of the system was allowed to reach local minima by mineral precipitation, however redox reactions among carbon and sulfur species was suppressed with a custom thermodynamic database. The resulting fluid compositions were used to calculate metabolic reaction energetics as well as to evaluate the potential effects of metal speciation on sulfate reduction rates.
BCO-DMO Data Processing Notes:
-reformatted column names to comply with BCO-DMO standards
-filled in all blank cells with nd
-removed spaces and replaced with underscores
| File |
|---|
SRenergetics.csv (Comma Separated Values (.csv), 9.22 KB) MD5:e2f36ee08e22a8c6342e99106925619b Primary data file for dataset ID 661659 |
| Parameter | Description | Units |
| experiment_num | PI issued experiment ID number | unitless |
| sulfide | Independently varied concentration of sulfide | molar (M) |
| sulfate | Independently varied concentration of sulfate | millimoles (mM) |
| DOC | Independently varied dissolved oxygen concentration | micromoles (uM) |
| citrate | Citrate concentration which made up DOC | molar (M) |
| formate | Formate concentration which made up DOC | molar (M) |
| pyruvate | Pyruvate concentration which made up DOC | molar (M) |
| acetate | Acetate concentration which made up DOC | molar (M) |
| lactate | Lactate concentration which made up DOC | molar (M) |
| bicarbonate | Concentration of bicarbonate which made up artificial seawater mix | millimoles (mM) |
| K | Concentration of K which made up artificial seawater mix | molar (M) |
| Ca | Concentration of Ca which made up artificial seawater mix | molar (M) |
| Na | Concentration of Na which made up artificial seawater mix | molar (M) |
| Cl | Concentration of Cl which made up artificial seawater mix | molar (M) |
| pH | pH of media added to incubations; Incubations were carried out at either pH 4 or 6; 4: simulates the pH of end-member Grotto vent fluid and the average calculated pH of mixed fluids in highly reduced zones within flange (Tivey 2004). 6: represents the calculated pH in fluid mixing zones (Tivey 2004). | pH |
| temperature | Temperatures at which samples were incubated anaerobically for 1 3 or 7 days. 4 C: ambient seawater; 50 C: thermophilic; 90 C: hyperthermophilic. | celsius (C) |
| sulfateRed_formate | (DeltaGr KJ/mol e-) of formate calculated at pH 6 and 50 deg C for heterotrophic sulfate reduction via organic acids | DeltaGr KJ/mol e- |
| sulfateRed_acetate | (DeltaGr KJ/mol e-) of acetate calculated at pH 6 and 50 deg C for heterotrophic sulfate reduction via organic acids | DeltaGr KJ/mol e- |
| sulfateRed_pyruvate | (DeltaGr KJ/mol e-) of pyruvate calculated at pH 6 and 50 deg C for heterotrophic sulfate reduction via organic acids | DeltaGr KJ/mol e- |
| sulfateRed_lactate | (DeltaGr KJ/mol e-) of lactate calculated at pH 6 and 50 deg C for heterotrophic sulfate reduction via organic acids | DeltaGr KJ/mol e- |
| sulfateRed_citrate | (DeltaGr KJ/mol e-) of citrate calculated at pH 6 and 50 deg C for heterotrophic sulfate reduction via organic acids | DeltaGr KJ/mol e- |
| c_limiting_totalEnergy | Carbon limiting total energy available in bottle for sulfate reduction | joules (J) |
| S_limiting_totalEnergy | Sulfate limiting total energy available in bottle for sulfate reduction | joules (J) |
| total_energy | Total energy available for sulfate reduction | joules (J) |
| lat | Latitude | decimal degrees |
| lon | Longitude | decimal degrees |
| Dataset-specific Instrument Name | Incubator |
| Generic Instrument Name | In-situ incubator |
| Dataset-specific Description | Used aboard ship and in lab |
| Generic Instrument Description | A device on a ship or in the laboratory that holds water samples under controlled conditions of temperature and possibly illumination. |
| Dataset-specific Instrument Name | DO sensor |
| Generic Instrument Name | Oxygen Sensor |
| Dataset-specific Description | DOC was measured |
| Generic Instrument Description | An electronic device that measures the proportion of oxygen (O2) in the gas or liquid being analyzed |
| Dataset-specific Instrument Name | pH sensor |
| Generic Instrument Name | pH Sensor |
| Dataset-specific Description | pH sensor |
| Generic Instrument Description | An instrument that measures the hydrogen ion activity in solutions.
The overall concentration of hydrogen ions is inversely related to its pH. The pH scale ranges from 0 to 14 and indicates whether acidic (more H+) or basic (less H+). |
AT18-08
| Website | |
| Platform | R/V Atlantis |
| Report | |
| Start Date | 2011-07-19 |
| End Date | 2011-08-01 |
| Description | Data expected from C-DEBI investigator, Julie Huber.
Additional cruise information and original data are available from the NSF R2R data catalog. |
AT18-08_Jason_Dives
| Website | |
| Platform | R/V Atlantis |
| Start Date | 2011-07-21 |
| End Date | 2011-07-31 |
Characterizing the distribution and rates of microbial sulfate reduction at Middle Valley hydrothermal vents (Middle Valley Vents)
This project characterizes rates of microbially mediated sulfate reduction from three distinct hydrothermal vents in the Middle Valley vent field along the Juan de Fuca Ridge, as well as assessments of bacterial and archaeal diversity, estimates of total biomass and the abundance of functional genes related to sulfate reduction, and in situ geochemistry. Maximum rates of sulfate reduction occurred at 90°C in all three deposits. Pyrosequencing and functional gene abundance data reveal differences in both biomass and community composition among sites, including differences in the abundance of known sulfate reducing bacteria. The abundance of sequences for Thermodesulfovibro-like organisms and higher sulfate reduction rates at elevated temperatures, suggests that Thermodesulfovibro-like organisms may play a role in sulfate reduction in warmer environments. The rates of sulfate reduction observed suggest that - within anaerobic niches of hydrothermal deposits - heterotrophic sulfate reduction may be quite common and might contribute substantially to secondary productivity, underscoring the potential role of this process in both sulfur and carbon cycling at vents.
This project was funded, in part, by a C-DEBI Graduate Student Fellowship.
Center for Dark Energy Biosphere Investigations (C-DEBI)
The mission of the Center for Dark Energy Biosphere Investigations (C-DEBI) is to explore life beneath the seafloor and make transformative discoveries that advance science, benefit society, and inspire people of all ages and origins.
C-DEBI provides a framework for a large, multi-disciplinary group of scientists to pursue fundamental questions about life deep in the sub-surface environment of Earth. The fundamental science questions of C-DEBI involve exploration and discovery, uncovering the processes that constrain the sub-surface biosphere below the oceans, and implications to the Earth system. What type of life exists in this deep biosphere, how much, and how is it distributed and dispersed? What are the physical-chemical conditions that promote or limit life? What are the important oxidation-reduction processes and are they unique or important to humankind? How does this biosphere influence global energy and material cycles, particularly the carbon cycle? Finally, can we discern how such life evolved in geological settings beneath the ocean floor, and how this might relate to ideas about the origin of life on our planet?
C-DEBI's scientific goals are pursued with a combination of approaches:
(1) coordinate, integrate, support, and extend the research associated with four major programs—Juan de Fuca Ridge flank (JdF), South Pacific Gyre (SPG), North Pond (NP), and Dorado Outcrop (DO)—and other field sites;
(2) make substantial investments of resources to support field, laboratory, analytical, and modeling studies of the deep subseafloor ecosystems;
(3) facilitate and encourage synthesis and thematic understanding of submarine microbiological processes, through funding of scientific and technical activities, coordination and hosting of meetings and workshops, and support of (mostly junior) researchers and graduate students; and
(4) entrain, educate, inspire, and mentor an interdisciplinary community of researchers and educators, with an emphasis on undergraduate and graduate students and early-career scientists.
Note: Katrina Edwards was a former PI of C-DEBI; James Cowen is a former co-PI.
Data Management:
C-DEBI is committed to ensuring all the data generated are publically available and deposited in a data repository for long-term storage as stated in their Data Management Plan (PDF) and in compliance with the NSF Ocean Sciences Sample and Data Policy. The data types and products resulting from C-DEBI-supported research include a wide variety of geophysical, geological, geochemical, and biological information, in addition to education and outreach materials, technical documents, and samples. All data and information generated by C-DEBI-supported research projects are required to be made publically available either following publication of research results or within two (2) years of data generation.
To ensure preservation and dissemination of the diverse data-types generated, C-DEBI researchers are working with BCO-DMO Data Managers make data publicly available online. The partnership with BCO-DMO helps ensure that the C-DEBI data are discoverable and available for reuse. Some C-DEBI data is better served by specialized repositories (NCBI's GenBank for sequence data, for example) and, in those cases, BCO-DMO provides dataset documentation (metadata) that includes links to those external repositories.
| Funding Source | Award |
|---|---|
| NSF Division of Ocean Sciences (NSF OCE) | |
| NSF Division of Ocean Sciences (NSF OCE) | |
| NASA Astrobiology Science & Technology for Exploring Planets (NASA-ASTEP) | |
| NASA Astrobiology Science & Technology for Exploring Planets (NASA-ASTEP) |
[ table of contents | back to top ]