Coral (Porites rus) calcification and chemistry data from outdoor flumes at the UCB Gump Research Station Moorea, French Polynesia in April and March of 2012
Project
Programs
| Contributors | Affiliation | Role |
|---|---|---|
| Carpenter, Robert | California State University Northridge (CSUN) | Principal Investigator |
| Comeau, Steeve | California State University Northridge (CSUN) | Co-Principal Investigator, Contact |
| Edmunds, Peter J. | California State University Northridge (CSUN) | Co-Principal Investigator |
| Srednick, Griffin | California State University Northridge (CSUN) | Technician |
| York, Amber D. | Woods Hole Oceanographic Institution (WHOI BCO-DMO) | BCO-DMO Data Manager |
Abstract
Methodology:
Calcification was estimated by measuring the change in buoyant weight (Davies 1989) based on an initial measurement of all the corals and subsequent measures on one-third of the corals after 1, 2, and 3 weeks of incubation in order to monitor the evolution of the treatment effects through time. The difference between initial and final buoyant weight was converted to dry weight increments using an aragonite density of 2.93 g cm-3 (Davies 1989) and standardized to the area of the corals as determined by the aluminum foil technique (Marsh 1970). Dry tissue weight of the organisms also was measured to normalize calcification to biomass to evaluate changes in biomass that might result from changes in the size of energy reserves attributed to the feeding regimes. To determine tissue dry weight, corals were fixed in 10 % formalin solution for 48 h, then the skeleton was dissolved by immersion in 5 % HCl that was replaced daily until the skeleton was dissolved (2–4 days). Tissues were rinsed in distilled water and dried for 48 h at 60 °C prior to weighing (±1 mg) and normalizing to area (mg cm-2).
pH was measured using an open-cell autotitrator (Model T50, Mettler-Toledo) calibrated every other day with Tris buffer provided by Dr. Andrew Dickson (Scripps Institution of Oceanography). Total alkalinity (AT) and salinity were measured daily during the first half of the incubation, and then every other day during the second half of the experiment based on the rationale that conditions were demonstrably stable. Seawater analyses were performed on the day of sampling using open-cell potentiometric titration with an automatic titrator (T50, Mettler-Toledo). Measurements were taken on 50-mL samples at *23 °C, and AT calculated after Dickson et al. (2007). Prior to each set of AT measurements, titrations of certified reference material (batch 108) provided by Dr. A. Dickson were performed and yielded values that were ±3 lmol kg-1 of certified values. Parameters of the carbonate system were calculated from salinity, temperature, AT, and pHT using the R package seacarb (Lavigne and Gattuso 2011).
See Comeau et al. (2013) for more details.
BCO-DMO Data Manager Processing Notes:
* Data submitted as sheet "data" in original excel file "comeau et al. 2013_data.xlsx" exported as csv with the formatting that was set in Excel.
* added a conventional header with dataset name, PI name, version date
* modified parameter names to conform with BCO-DMO naming conventions: only A-Za-z0-9 and underscore allowed. Can not start with a number. (spaces, +, and - changed to underscores).
* Duplicate column names given either suffix _1 or suffix _2. e.g. (DIC_1, DIC_2)
* blank values in this dataset are displayed as "nd" for "no data." nd is the default missing data identifier in the BCO-DMO system.
* Various date formats in Date column changed from to yyyy-mm-dd (e.g. 2015-11-13).
| File |
|---|
comeau2013_calcif.csv (Comma Separated Values (.csv), 37.88 KB) MD5:40472b3776f88bb860855a21f9878c44 Primary data file for dataset ID 754661 |
| Parameter | Description | Units |
| Species | Species identification in format Genus species (coral). | unitless |
| Date | Date | unitless |
| Treat | Treatment | unitless |
| Sample_label | Sample code/label | unitless |
| E | Irradiance | umol/m2/s |
| Calc_rate_CaCO3_mg_cm2_day | Calcification rate of calcium carbonate (mg/cm2/day). Buoyant weighing technique (Davies, 1989). | mg/cm2/day |
| Calc_rate_CaCO3_mg_mg_day | Calcification rate of calcium carbonate (mg/mg/day). Buoyant weighing technique (Davies, 1989). | mg/mg/day |
| biomass | biomass | mg/cm2 |
| Sal | Salinity | PSU |
| Temp | Temperature, water | degrees Celcius |
| pH | pH, Potentiometric. | total hydrogen ion scale (pHT) |
| CO2_1 | Carbon dioxide. Calculated using seacarb (Lavigne and Gattuso, 2011). | umol/kg |
| pCO2water_SST_wet_1 | Partial pressure of carbon dioxide (water) at sea surface temperature (wet air). Calculated using seacarb (Lavigne and Gattuso, 2011). | uatm |
| fCO2water_SST_wet_1 | Fugacity of carbon dioxide (water) at sea surface temperature (wet air). Calculated using seacarb (Lavigne and Gattuso, 2011). | uatm |
| Bicarbonate_ion_1 | Bicarbonate ion [HCO3]-. Calculated using seacarb (Lavigne and Gattuso, 2011). | umol/kg |
| Carbonate_ion_1 | Carbonate ion [CO3]2-. Calculated using seacarb (Lavigne and Gattuso, 2011). | umol/kg |
| DIC_1 | Carbon, inorganic, dissolved. Calculated using seacarb (Lavigne and Gattuso, 2011). | umol/kg |
| AT | Alkalinity, total. Potentiometric titration. | umol/kg |
| Omega_Arg_1 | Aragonite saturation state. Calculated using seacarb (Lavigne and Gattuso, 2011). | omega aragonite (Ωa) |
| Omega_Cal_1 | Calcite saturation state. Calculated using seacarb (Lavigne and Gattuso, 2011). | omega calcite (Ωcal) |
| CSC_flag | Carbonate system computation flag | unitless |
| CO2_2 | Carbon dioxide. Calculated using seacarb (Lavigne and Gattuso, 2011) after Nisumaa et al. (2010). | umol/kg |
| pCO2water_SST_wet_2 | Partial pressure of carbon dioxide (water) at sea surface temperature (wet air). Calculated using seacarb (Lavigne and Gattuso, 2011) after Nisumaa et al. (2010). | uatm |
| fCO2water_SST_wet_2 | Fugacity of carbon dioxide (water) at sea surface temperature (wet air). Calculated using seacarb (Lavigne and Gattuso, 2011) after Nisumaa et al. (2010). | uatm |
| Bicarbonate_ion_2 | Bicarbonate ion [HCO3]-. Calculated using seacarb (Lavigne and Gattuso, 2011) after Nisumaa et al. (2010). | umol/kg |
| Carbonate_ion_2 | Carbonate ion [CO3]2-. Calculated using seacarb (Lavigne and Gattuso, 2011) after Nisumaa et al. (2010). | umol/kg |
| DIC_2 | Carbon, inorganic, dissolved (DIC). Calculated using seacarb (Lavigne and Gattuso, 2011) after Nisumaa et al. (2010). | umol/kg |
| Omega_Arg_2 | Aragonite saturation state. Calculated using seacarb (Lavigne and Gattuso, 2011) after Nisumaa et al. (2010). | omega aragonite (Ωa) |
| Omega_Cal_2 | Calcite saturation state. Calculated using seacarb (Lavigne and Gattuso, 2011) after Nisumaa et al. (2010). | omega calcite (Ωcal) |
| Dataset-specific Instrument Name | 4p quantum sensor (LI-193) and a LiCor LI-1400 meter |
| Generic Instrument Name | Light Meter |
| Generic Instrument Description | Light meters are instruments that measure light intensity. Common units of measure for light intensity are umol/m2/s or uE/m2/s (micromoles per meter squared per second or microEinsteins per meter squared per second). (example: LI-COR 250A) |
| Dataset-specific Instrument Name | Orion 3-stars pH Meter fitted with a DG 115-SC pH probe |
| Generic Instrument Name | pH Sensor |
| Generic Instrument Description | An instrument that measures the hydrogen ion activity in solutions.
The overall concentration of hydrogen ions is inversely related to its pH. The pH scale ranges from 0 to 14 and indicates whether acidic (more H+) or basic (less H+). |
| Dataset-specific Instrument Name | YSI 3100 |
| Generic Instrument Name | Salinity Sensor |
| Generic Instrument Description | Category of instrument that simultaneously measures electrical conductivity and temperature in the water column to provide temperature and salinity data. |
| Dataset-specific Instrument Name | Mettler Toledo T50 |
| Generic Instrument Name | Titrator |
| Dataset-specific Description | TA: Mettler Toledo T50 |
| Generic Instrument Description | Titrators are instruments that incrementally add quantified aliquots of a reagent to a sample until the end-point of a chemical reaction is reached. |
| Dataset-specific Instrument Name | ThermoFisher Traceable |
| Generic Instrument Name | Water Temperature Sensor |
| Generic Instrument Description | General term for an instrument that measures the temperature of the water with which it is in contact (thermometer). |
Moorea Coral Reef Long-Term Ecological Research site (MCR LTER)
From http://www.lternet.edu/sites/mcr/ and http://mcr.lternet.edu/:
The Moorea Coral Reef LTER site encompasses the coral reef complex that surrounds the island of Moorea, French Polynesia (17°30'S, 149°50'W). Moorea is a small, triangular volcanic island 20 km west of Tahiti in the Society Islands of French Polynesia. An offshore barrier reef forms a system of shallow (mean depth ~ 5-7 m), narrow (~0.8-1.5 km wide) lagoons around the 60 km perimeter of Moorea. All major coral reef types (e.g., fringing reef, lagoon patch reefs, back reef, barrier reef and fore reef) are present and accessible by small boat.
The MCR LTER was established in 2004 by the US National Science Foundation (NSF) and is a partnership between the University of California Santa Barbara and California State University, Northridge. MCR researchers include marine scientists from the UC Santa Barbara, CSU Northridge, UC Davis, UC Santa Cruz, UC San Diego, CSU San Marcos, Duke University and the University of Hawaii. Field operations are conducted from the UC Berkeley Richard B. Gump South Pacific Research Station on the island of Moorea, French Polynesia.
MCR LTER Data: The Moorea Coral Reef (MCR) LTER data are managed by and available directly from the MCR project data site URL shown above. The datasets listed below were collected at or near the MCR LTER sampling locations, and funded by NSF OCE as ancillary projects related to the MCR LTER core research themes.
This project is supported by continuing grants with slight name variations:
- LTER: Long-Term Dynamics of a Coral Reef Ecosystem
- LTER: MCR II - Long-Term Dynamics of a Coral Reef Ecosystem
- LTER: MCR IIB: Long-Term Dynamics of a Coral Reef Ecosystem
- LTER: MCR III: Long-Term Dynamics of a Coral Reef Ecosystem
- LTER: MCR IV: Long-Term Dynamics of a Coral Reef Ecosystem
RUI: Ocean Acidification- Category 1- The effects of ocean acidification on the organismic biology and community ecology of corals, calcified algae, and coral reefs (OA_Corals)
While coral reefs have undergone unprecedented changes in community structure in the past 50 y, they now may be exposed to their gravest threat since the Triassic. This threat is increasing atmospheric CO2, which equilibrates with seawater and causes ocean acidification (OA). In the marine environment, the resulting decline in carbonate saturation state (Omega) makes it energetically less feasible for calcifying taxa to mineralize; this is a major concern for coral reefs. It is possible that the scleractinian architects of reefs will cease to exist as a mineralized taxon within a century, and that calcifying algae will be severely impaired. While there is a rush to understand these effects and make recommendations leading to their mitigation, these efforts are influenced strongly by the notion that the impacts of pCO2 (which causes Omega to change) on calcifying taxa, and the mechanisms that drive them, are well-known. The investigators believe that many of the key processes of mineralization on reefs that are potentially affected by OA are only poorly known and that current knowledge is inadequate to support the scaling of OA effects to the community level. It is vital to measure organismal-scale calcification of key taxa, elucidate the mechanistic bases of these responses, evaluate community scale calcification, and finally, to conduct focused experiments to describe the functional relationships between these scales of mineralization.
This project is a 4-y effort focused on the effects of Ocean Acidification (OA) on coral reefs at multiple spatial and functional scales. The project focuses on the corals, calcified algae, and coral reefs of Moorea, French Polynesia, establishes baseline community-wide calcification data for the detection of OA effects on a decadal-scale, and builds on the research context and climate change focus of the Moorea Coral Reef LTER.
This project is a hypothesis-driven approach to compare the effects of OA on reef taxa and coral reefs in Moorea. The PIs will utilize microcosms to address the impacts and mechanisms of OA on biological processes, as well as the ecological processes shaping community structure. Additionally, studies of reef-wide metabolism will be used to evaluate the impacts of OA on intact reef ecosystems, to provide a context within which the experimental investigations can be scaled to the real world, and critically, to provide a much needed reference against which future changes can be gauged.
Datasets listed in the "Dataset Collection" section include references to results journal publications published as part of this project.
Long Term Ecological Research network (LTER)
adapted from http://www.lternet.edu/
The National Science Foundation established the LTER program in 1980 to support research on long-term ecological phenomena in the United States. The Long Term Ecological Research (LTER) Network is a collaborative effort involving more than 1800 scientists and students investigating ecological processes over long temporal and broad spatial scales. The LTER Network promotes synthesis and comparative research across sites and ecosystems and among other related national and international research programs. The LTER research sites represent diverse ecosystems with emphasis on different research themes, and cross-site communication, network publications, and research-planning activities are coordinated through the LTER Network Office.
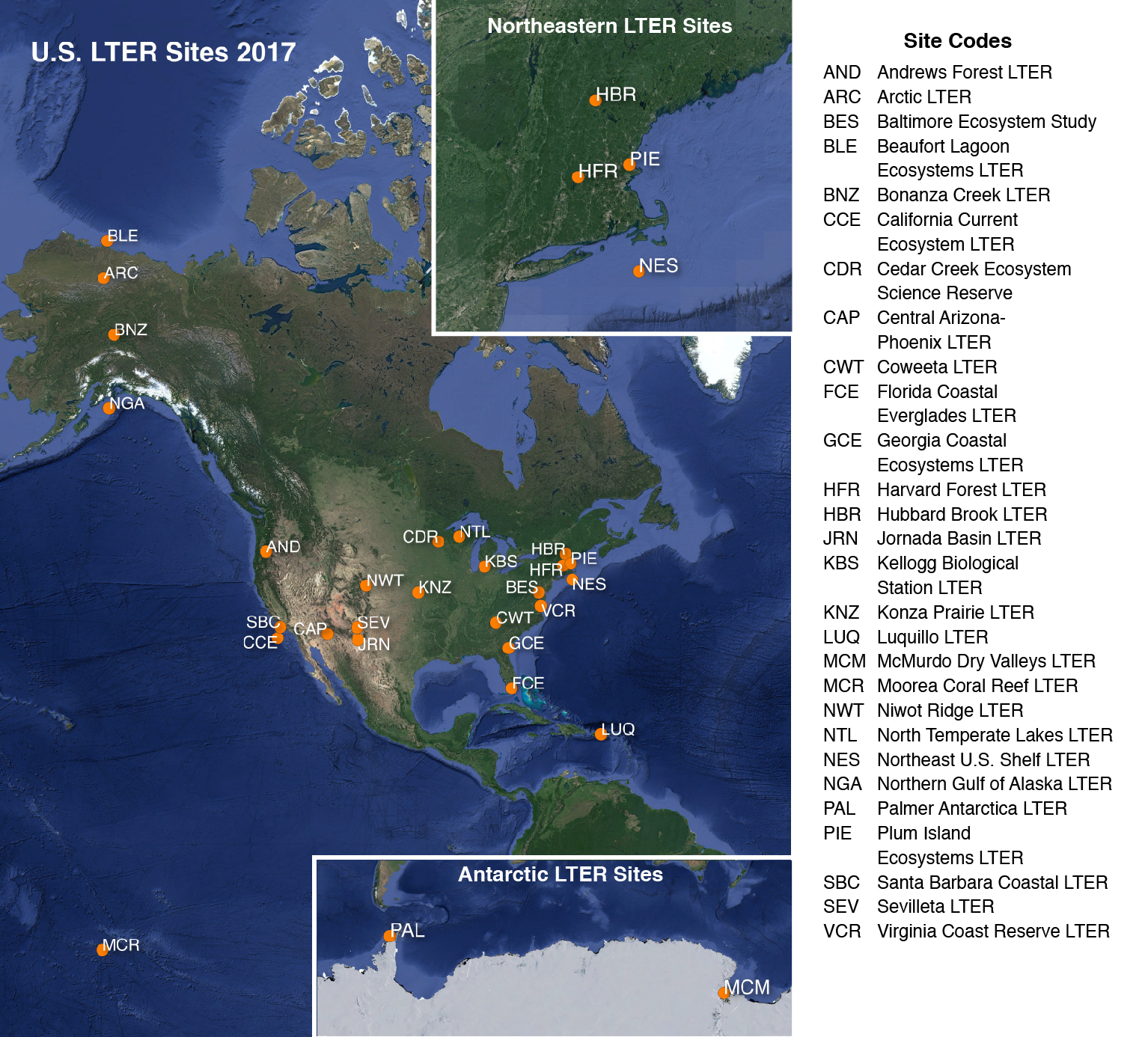
2017 LTER research site map obtained from https://lternet.edu/site/lter-network/
Science, Engineering and Education for Sustainability NSF-Wide Investment (SEES): Ocean Acidification (formerly CRI-OA) (SEES-OA)
NSF Climate Research Investment (CRI) activities that were initiated in 2010 are now included under Science, Engineering and Education for Sustainability NSF-Wide Investment (SEES). SEES is a portfolio of activities that highlights NSF's unique role in helping society address the challenge(s) of achieving sustainability. Detailed information about the SEES program is available from NSF (https://www.nsf.gov/funding/pgm_summ.jsp?pims_id=504707).
In recognition of the need for basic research concerning the nature, extent and impact of ocean acidification on oceanic environments in the past, present and future, the goal of the SEES: OA program is to understand (a) the chemistry and physical chemistry of ocean acidification; (b) how ocean acidification interacts with processes at the organismal level; and (c) how the earth system history informs our understanding of the effects of ocean acidification on the present day and future ocean.
Solicitations issued under this program:
NSF 10-530, FY 2010-FY2011
NSF 12-500, FY 2012
NSF 12-600, FY 2013
NSF 13-586, FY 2014
NSF 13-586 was the final solicitation that will be released for this program.
PI Meetings:
1st U.S. Ocean Acidification PI Meeting(March 22-24, 2011, Woods Hole, MA)
2nd U.S. Ocean Acidification PI Meeting(Sept. 18-20, 2013, Washington, DC)
3rd U.S. Ocean Acidification PI Meeting (June 9-11, 2015, Woods Hole, MA – Tentative)
NSF media releases for the Ocean Acidification Program:
Press Release 10-186 NSF Awards Grants to Study Effects of Ocean Acidification
Discovery Blue Mussels "Hang On" Along Rocky Shores: For How Long?
Press Release 13-102 World Oceans Month Brings Mixed News for Oysters
| Funding Source | Award |
|---|---|
| NSF Division of Ocean Sciences (NSF OCE) | |
| NSF Division of Ocean Sciences (NSF OCE) | |
| NSF Division of Ocean Sciences (NSF OCE) |
[ table of contents | back to top ]