Trial A test of the dissolution method for estimates of the 15N2 atom% of incubations
Project
| Contributors | Affiliation | Role |
|---|---|---|
| Granger, Julie | University of Connecticut (UConn) | Principal Investigator |
| Bourbonnais, Annie | University of Massachusetts Dartmouth (UMass Dartmouth) | Co-Principal Investigator |
| Wilson, Samuel | University of Hawai'i (UH) | Co-Principal Investigator |
| Biddle, Mathew | Woods Hole Oceanographic Institution (WHOI BCO-DMO) | BCO-DMO Data Manager |
Abstract
Trial A test of the dissolution method
Inocula of 15N2-enriched water were prepared according to either of two protocols outlined by Klawonn et al. (2015).
In a first Trial A, respective 1.9 mL of 15N2 gas aliquots (Cambridge Isotope Laboratories, Lot #I-21065) were injected into crimped-sealed 120 mL glass serum vials filled with deionized water. To dissolve the 15N2 bubble, each of the two serum vials was vortexed for 5 minutes. Two subsamples of each inoculum were dispensed into Exetainers™ with a peristaltic pump for analysis on the MIMS. An aliquot of each inoculum (5 % vol/vol) was then dispensed in replicate 160 mL serum incubation bottles containing air-equilibrated deionized water (Trials A1-A4), which were then crimped-sealed. Following homogenization, triplicate subsamples of each incubation were collected in Exetainers™ for MIMS analysis. The 15N atom % of the inocula and of the corresponding incubations were measured by MIMS at the University of Connecticut (Bay Instruments) and computed as follows:
Equation 4: 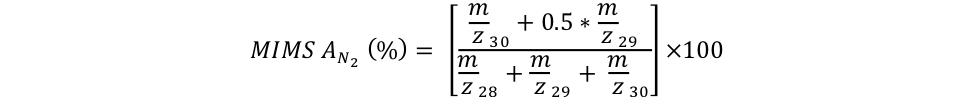
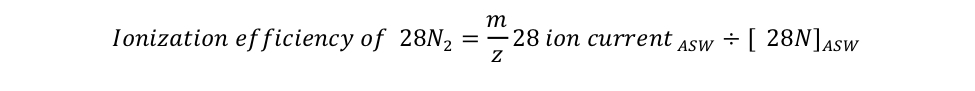
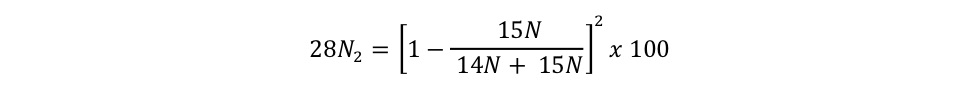
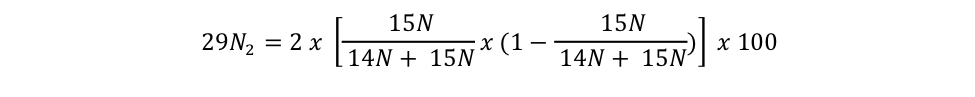 = 0.7299 % Equation S3b
= 0.7299 % Equation S3b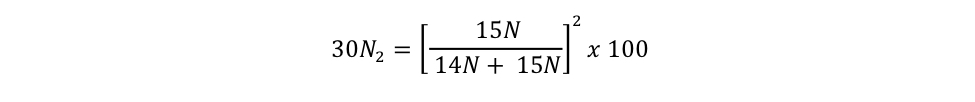 = 0.0013 % Equation S3c
= 0.0013 % Equation S3cBCO-DMO Processing Notes:
- table was extracted from original spreadsheet.
- added conventional header with dataset name, PI name, version date
- modified parameter names to conform with BCO-DMO naming conventions
| File |
|---|
trial_a.csv (Comma Separated Values (.csv), 6.16 KB) MD5:2298e8da89522e950fdb4b6cf8296398 Primary data file for dataset ID 778126 |
| File |
|---|
equation 4 EAGER_NitFix filename: equation_4.jpg (JPEG Image (.jpg), 24.62 KB) MD5:25f19b478991407bd347bd9221c4857b The 15N atom % of the inocula and of the corresponding incubations were measured by MIMS at the University of Connecticut (Bay Instruments) and computed using this equation. |
equation S2 Eager_NitFix filename: equation_s2.jpg (JPEG Image (.jpg), 23.66 KB) MD5:af88aa623a8e948669b26dbeb4dbaeaf Equation for the definition of the ionization efficiency as the ratio of the isotopologue ion current measured by MIMS relative to its concentration in air-equilibrated seawater (ASW). |
equation s3a Eager_NitFix filename: equation_s3a.jpg (JPEG Image (.jpg), 9.58 KB) MD5:fdc41b085863b05134fbb25f6cc25c54 Equation for the binomial probability distribution for 28N2. |
equation s3b Eager_NitFix filename: equation_s3b.jpg (JPEG Image (.jpg), 22.12 KB) MD5:d7200880be28043d090d28bebeda9d12 Equation for the binomial probability distribution for 29N2. |
equation s3c Eager_NitFix filename: equation_s3c.jpg (JPEG Image (.jpg), 14.71 KB) MD5:04c7942d3273c43e9c10e3ed2b41b0fd Equation for the binomial probability distribution for 30N2. |
| Parameter | Description | Units |
| Sample | sample | unitless |
| Baro_Press | barometric pressue | unknown |
| Time_of_analysis | time of analysis | unitless |
| m_z_28 | mass-to-charge | unitless |
| m_z_29 | mass-to-charge | unitless |
| m_z_30 | mass-to-charge | unitless |
| m_z_32 | mass-to-charge | unitless |
| m_z_40 | mass-to-charge | unitless |
| N2_Ar | N2/Ar ratio | unitless |
| ratio_28_29 | 28/29 ratio | unitless |
| ratio_28_30 | 28/30 ratio | unitless |
| meas_at_pcnt | measured atom percent | unitless |
| avg_measured_a_pcnt | average measured atom percent | unitless |
| Dataset-specific Instrument Name | Isotope Ratio Mass Spectrometer |
| Generic Instrument Name | Isotope-ratio Mass Spectrometer |
| Dataset-specific Description | continuous flow Delta V Isotope Ratio Mass Spectrometer (Smith et al. 2015), and continuous flow-GV Isoprime IRMS (Charoenpong et al., 2014) |
| Generic Instrument Description | The Isotope-ratio Mass Spectrometer is a particular type of mass spectrometer used to measure the relative abundance of isotopes in a given sample (e.g. VG Prism II Isotope Ratio Mass-Spectrometer). |
| Dataset-specific Instrument Name | Membrane Inlet Mass Spectrometer |
| Generic Instrument Name | Membrane Inlet Mass Spectrometer |
| Dataset-specific Description | Membrane Inlet Mass Spectrometer (Bay Instruments) |
| Generic Instrument Description | Membrane-introduction mass spectrometry (MIMS) is a method of introducing analytes into the mass spectrometer's vacuum chamber via a semipermeable membrane. |
EAGER: Collaborative Research: Detection limit in marine nitrogen fixation measurements - Constraints of rates from the mesopelagic ocean (EAGER NitFix)
NSF Award Abstract:
The availability of nitrogen is required to support the growth and production of organisms living in the surface of our global ocean. This element can be scarce. To alleviate this scarcity, a special class of bacteria and archaea, called nitrogen fixers, can derive the nitrogen needed for growth from nitrogen gas. This project would carefully examine one specific method for measuring nitrogen fixation that has been used recently to suggest the occurrence of small amounts of nitrogen fixation in subsurface ocean waters. If these reports are verified, then a revision of our understanding of the marine nitrogen cycle may be needed. The Ocean Carbon and Biogeochemistry program will be used as a platform to develop community consensus for best practices in nitrogen fixation measurements and detection of diversity, activity, and abundances of the organisms responsible. In addition, a session will be organized in a future national/international conference to communicate with the broader scientific community while developing these best practices.
The goal of this study is to conduct a thorough examination of potential experimental and analytical errors inherent to the 15N2-tracer nitrogen fixation method, in tandem with comprehensive molecular measurements, in mesopelagic ocean waters. Samples will be collected and experimental work conducted on a cruise transect in the North Atlantic Ocean, followed by analytical work in the laboratory. The specific aims of this study are to (1) determine the minimum quantifiable rates of 15N2 fixation based on incubations of mesopelagic waters via characterization of sources of experimental and analytical error, and (2) seek evidence of presence and expression of nitrogen fixation genes via comprehensive molecular approaches on corresponding samples. The range of detectable rates and diazotroph activity from the measurements made in this study will be informative for the understanding of the importance of nitrogen fixation in the oceanic nitrogen budget.
| Funding Source | Award |
|---|---|
| NSF Division of Ocean Sciences (NSF OCE) |
[ table of contents | back to top ]