The DISC is a free-floating, cylindrical behavioral observation chamber composed of clear acrylic and is used to monitor the behavior of marine larvae in situ. During each trial, an individual fish larva is placed inside of the central arena (20 cm diameter, 10 cm height) which is transparent to odor, light, and sound. The bottom of the arena is made of clear plexiglass, while the top is made of a fine mesh, and the walls are made of a black opaque film. Larvae can swim freely inside of the arena, and their behavior is recorded using a camera system which is supplemented by information on the rotation of the DISC and by records of the environment (temperature, light intensity, salinity, GPS).
A reference context was first defined. This was the ecological context in which we imagined a larva would be exposed to the most information. For this set of experiments, larvae were deployed in the DISC to a depth of 18 meters, within a kilometer from the reef, and during the ebb tide. The depth was chosen to reduce light scattering / intensity and based on observations of the vertical distribution of similar goby species. The distance was chosen to expose the larvae to higher sound levels and more concentrated chemosensory stimulants. The ebb tide was chosen to create a link between flow direction and chemosensory information, as we suspected that the ebb current would come from the direction of the reef.
To test the importance of the different environmental variables, three additional ecological contexts were defined by varying one of the defining features of the reference context at a time. That is, one group (shallow context) of larvae was deployed at 9 meters, within a kilometer from the reef, and during the ebb tide. One group (far context) was deployed at 18 meters, further than a kilometer from the reef, and during the ebb tide. The final group (flood context) was deployed at 18 meters, within a kilometer from the reef, and during the flood tide. For group-level analysis, significantly-directional individuals in a common ecological context were pooled. They were then assessed for significant consistency in their mean angles using a second-order Rayleigh’s test. Additionally, the precision of orientation (rho-value) was compared between ecological contexts, as was the mean larval turning angle (i.e., the angle produced by three successive points on a larva’s trajectory). Finally, the percentage of orienting individuals in each context was compared using a logistic model. In addition to looking at differences in circular statistics among contexts, we also assessed whether there was evidence of several common orientation hypotheses, both within and across contexts. Specifically, we looked for evidence of a sun compass, orientation with the current direction, orientation with the wind direction, and orientation variation with temperature and light.
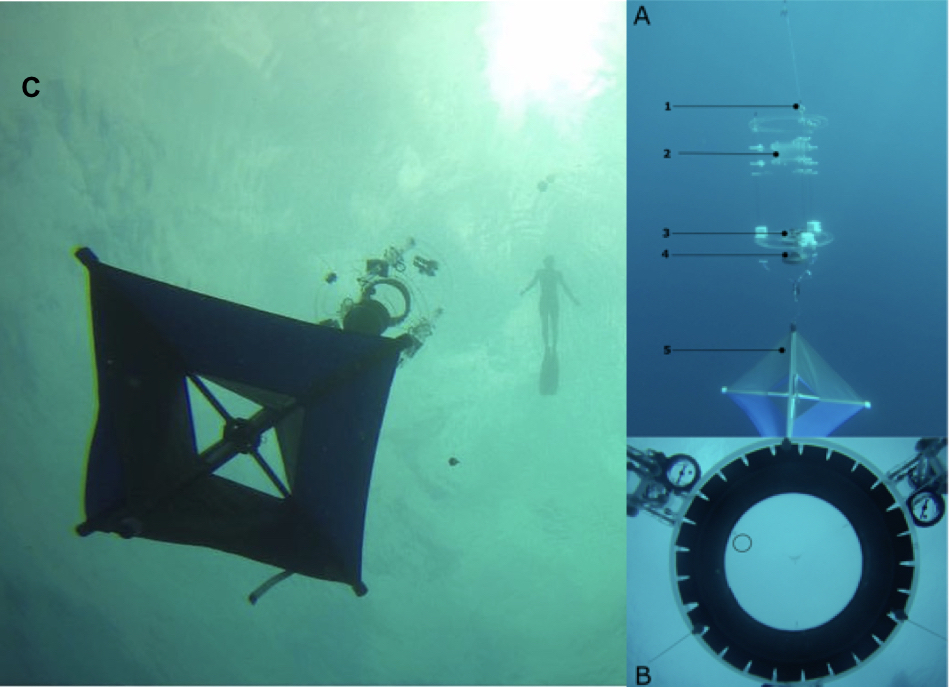
Figure 1. Drifting In Situ Chamber (DISC).
A. Schematic of DISC components. A thin kite-line (A1) tethers the DISC to a surface float equipped with a GPS and to a subsurface float that decouples the movement of the DISC in the water mass in which it is embedded from potential stokes drift. The clear acrylic DISC frame holds a behavioral arena (A2, 20.32 cm diameter, 10 cm height) - transparent to sound, light, and chemical cues – where individual larvae are deployed. A video camera (A3), placed 35 cm under the chamber, records the larval movement continuously during each 15-minute trial, which includes 5 minutes of acclimation time and 10 minutes of observation time. Environmental sensors (A4) attached to the DISC’s frame record light levels, temperature, salinity, depth, earth’s magnetic field strength, 3-D acceleration, and compass heading. The compass serves to track the DISC’s rotation and is used to translate the movement of the larva from the chamber’s frame of reference to a cardinal frame of reference. A drogue (A5) is attached to the DISC’s bottom frame, keeping the DISC locked in the instantaneous current at the depth of the instrument. For deep deployments, the DISC was initially deployed to 9 m and lowered to 18 m with a dive reel placed above the DISC’s tethering bridles. To optimize the number of observations, two DISCs were deployed simultaneously.
B. Example of image collected by the DISC’s camera; the location of the larva is indicated by the black circle. A series of these images are used to manually track the location of the larva inside the chamber throughout the deployment.
C. Deployment of the DISC at 18 m offshore the Belize Barrier Reef.

 ©2024 Biological and Chemical Oceanography Data Management Office.
©2024 Biological and Chemical Oceanography Data Management Office.
